Abstract
Activation of the Ca2+/calmodulin-dependent protein kinase II isoform δA (CaMKIIδA) disturbs intracellular Ca2+ homeostasis in cardiomyocytes during chronic heart failure (CHF). We hypothesized that upregulation of CaMKIIδA in cardiomyocytes might enhance Ca2+ leak from the sarcoplasmic reticulum (SR) via activation of phosphorylated ryanodine receptor type 2 (P-RyR2) and decrease Ca2+ uptake by inhibition of SR calcium ATPase 2a (SERCA2a). In this study, CHF was induced in rats by ligation of the left anterior descending coronary artery. We found that CHF caused an increase in the expression of CaMKIIδA and P-RyR2 in the left ventricle (LV). The role of CaMKIIδA in regulation of P-RyR2 was elucidated in cardiomyocytes isolated from neonatal rats in vitro. Hypoxia induced upregulation of CaMKIIδA and activation of P-RyR2 in the cardiomyocytes, which both were attenuated by knockdown of CaMKIIδA. Furthermore, we showed that knockdown of CaMKIIδA significantly decreased the Ca2+ leak from the SR elicited by hypoxia in the cardiomyocytes. In addition, CHF also induced a downregulation of SERCA2a in the LV of CHF rats. Knockdown of CaMKIIδA normalized hypoxia-induced downregulation of SERCA2a in cardiomyocytes in vitro. The results demonstrate that the inhibition of CaMKIIδA may improve cardiac function by preventing SR Ca2+ leak through downregulation of P-RyR2 and upregulation of SERCA2a expression in cardiomyocytes in CHF.
Similar content being viewed by others
Introduction
A disturbance in intracellular Ca2+ homeostasis has been well known to contribute to the cardiac dysfunction observed in chronic heart failure (CHF)1. Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ) is predominantly expressed in the heart2 and is alternatively spliced to generate three subtypes3. These CaMKIIδ isoforms are regulated by calcium-ligand binding calmodulin (Ca2+/CaM)2. The CaMKIIδA subtype has been found to be expressed in the heart4. The CaMKIIδB subtype is expressed in the heart and aorta, and the CaMKIIδC subtype is expressed in the brain, heart, aorta, liver and intestine under physiological conditions3,5. Additionally, studies have indicated that the expression of CaMKIIδ subtypes is altered during cardiomyocyte differentiation and maturation and in association with the development of CHF3.
CaMKIIδ regulates calcium homeostasis and cardiac function6. Expression of the phosphorylated ryanodine receptor type 2 (P-RyR2), the channel through which Ca2+ exits the SR, is upregulated in CaMKIIδC transgenic (TC) mice7. Hyper-phosphorylation of RyR2 by CaMKIIδC is expected to enhance the leak of Ca2+ from the SR because acute inhibition of CaMKII in CaMKIIδC TC myocytes has been shown to decrease SR Ca2+ leak8. Interestingly, CaMKIIδA expression is also increased in isoproterenol-induced cardiac hypertrophy, and thus, regulation of CaMKIIδ splicing may be relevant to cardiac function4. Therefore, regulation of CaMKIIδ splicing may account for altered subtype expression and signaling in both physiological and pathophysiological settings.
Accelerating diastolic Ca2+ release from the sarcoplasmic reticulum (SR) via phosphorylation of RyR2 provokes cardiac dysfunction in CHF9,10,11,12. Furthermore, CaMKII can also induce protein kinase A (PKA)-independent diastolic SR Ca2+ leak13,14,15. SERCA2a expression and activity are downregulated after myocardial ischemia in failing hearts16,17. Decreased SERCA2a was shown to result in impaired SR Ca2+ reuptake, which combined with leaky RyR2 to deplete SR Ca2+ and lead to impaired cardiac contractility. Therefore, restoration of normal levels of SERCA2a has been targeted as a novel therapeutic for CHF18,19. SERCA2a, similar to RyR2, is also regulated by CaMKII, which mediates calcium activity in the SR of cardiomyocytes20. The critical roles of CaMKIIδB and δC in CHF have been well investigated21,22,23. Recently, a study showed that CaMKIIδA, which selectively interferes with the histone deacetylase/myocyte enhancer factor-2 signaling pathway, is also critically involved in cardiac hypertrophy in both neonatal and adult models24.
A better understanding of the molecular mechanisms underlying the effects of CaMKIIδA on the regulation of intracellular Ca2+ homeostasis might facilitate the identification of novel therapeutic targets to improve cardiac function in CHF. Therefore, the purpose of the present study was to determine the molecular signaling mechanisms by which CaMKIIδA activation disturbs cytoplasmic Ca2+ homeostasis in cardiomyocytes with CHF.
Materials and methods
Animals and cardiomyocyte isolation
Experiments were carried out on 30 male Sprague Dawley (SD) rats weighing 200 to 220 g. The present study was approved by the Nantong University Council on Animal Care and Use and complied with the Guide for the Care and Use of Laboratory Animals. Animals were anesthetized by chloral hydrate (50 mg/kg ip). Artificial ventilation was used with oxygen-enriched room air. Tidal volume was 2.0 to 3.0 mL. After the heart was exposed through a lateral thoracotomy, the left anterior descending (LAD) coronary artery was ligated as we described previously25. Acute myocardial infarction was confirmed by the observation that the anterior wall of the left ventricle became cyanotic and through an elevation in the ST segment of the electrocardiogram (ECG, lead II) waveform. Three days later, acute heart failure was confirmed by echocardiography (data not shown here). Rats were randomly assigned into two groups: (1) sham controls, which received a lateral thoracotomy but no ligation of LAD artery, and (2) the CHF group (after 4 weeks of LAD artery ligation). Ventricular cardiomyocytes from 1 to 2-day-old neonatal rat hearts were prepared by trypsin digestion as described previously26. After 24 h, these cardiomyocytes were treated with hypoxia and transfected with CaMKIIδA siRNA or scrambled RNA.
Hypoxic culture condition
Hypoxia is used to model cardiovascular diseases because of the insufficient oxygen supply associated with these diseases. Hypoxia is a hazardous consequence of cardiac ischemia, which triggers a wide range of cellular responses27. Therefore, in the present study, the culture of neonatal cardiomyocytes under hypoxic conditions was used to mimic the insufficient oxygen supply induced by cardiac ischemia. Hypoxic conditions were performed as previously described28,29. Cells were incubated for 12 h with humidified gas containing 1% O2, 5% CO2 and 94% N2 (tri-gas incubator, Thermo ScientificTM). Control cells were grown in normal oxygen conditions for the same duration.
Intracellular Ca2+ imaging
Neonatal cardiomyocytes were plated in a 96-well plate. Cardiomyocytes were loaded with 1 μmol/L Fluo-4 (Invitrogen, USA) in extracellular buffer (in mmol/L, 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 Hepes) for 30 min at room temperature. Next, cells were washed with extracellular buffer and kept in this buffer until use. Fluo-4 was used to monitor cytosolic Ca2+ in cardiomyocytes. SR Ca2+ leak was measured by the tetracaine method as previously described30,31. Briefly, Fluo-4-loaded cardiomyocytes were paced at 0.5 Hz in normal Tyrode's solution (NT, 140 mmol/L NaCl, 4 mmol/L KCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L glucose, and 5 mmol/L HEPES, pH 7.4) for 20 s. The solution was then rapidly changed into 0 Na+, 0 Ca2+ NT (NT with no added Ca2+, 10 mmol/L EGTA, and 140 mmol/L LiCl substituted for NaCl, pH 7.4 with 4 mmol/L tetracaine). The tetracaine-induced intracellular Ca2+ drop was considered to be an index of SR Ca2+ leak32,33. Fluo-4 fluorescence was excited at 488 nm, and data were expressed as normalized changes in background-corrected fluorescence emission (F/F 0). Data were acquired using Leica SP2 confocal software (Leica Microsystems, Germany).
Western blot analysis
Protein lysates were subjected to separation on a 10% SDS-PAGE gel, followed by electrotransfer to nitrocellulose membranes. Blots were individually probed with specific antibodies against RyR2 (phospho S2814) (A010-31, Badrilla), SERCA2a (A010-20, Labome), and GAPDH (G9545, Sigma). Signals were detected by the enhanced chemiluminescence detection method and quantified by densitometry using Quality One software (Bio-Rad).
Quantitative real-time PCR analysis
Total RNA was extracted from the isolated left ventricle (LV) heart tissue from both groups of rats (n=5 for each group) using a total RNA isolation kit (Qiagen, Hilden, Germany). cDNA was prepared with a Superscript kit (Invitrogen, Tokyo, Japan) according to the manufacturer's instructions. Quantitative real-time PCR was performed as we described32. The oligonucleotide primer sequences were as follows: CaMKIIδA (sense: 5'--3' and antisense: 5'--3'), CaMKIIδB (sense: 5'--3' and antisense: 5'--3'), and CaMKIIδC (sense: 5'-3' and antisense: 5'--3'). GAPDH (sense: 5'--3' and antisense: 5'--3') was used as the internal standard4. Small interfering RNAs (siRNAs) targeting the CamkIIδA gene were synthesized by Biomics (Nantong, China), and the effect of the siRNA was identified by RT-PCR. The siRNA was then used in further experiments. The two siRNA sequences used were as follows: 5'--3' and 5'--3'24. Scrambled RNA oligonucleotides were used as a control. Twenty-four hours prior to transfection, cardiomyocytes were plated onto a 6-well plate (Corning Inc, Corning, NY, USA). For each well, 50 nmol/L of the three oligos were transfected using LipoD293 (SignaGen) according to the manufacturer's instructions. The medium was replaced with DMEM containing 10% fetal bovine serum (FBS) after 6 h.
Ultrasound cardiograph
Echocardiograms were obtained from animals (chloral hydrate, 50 mg/kg, ip) with a Vevo770 system (Visual Sonics Inc, Toronto, Canada) equipped with a 17.5 MHz transducer by an experienced technician who was blinded to the treatment groups. In brief, the rats were anesthetized (chloral hydrate, 50 mg/kg, ip), shaved of chest fur, and placed supine on a special table. M-mode tracings were recorded at baseline. The left ventricular internal diameter at the diastolic phase (LVIDd), LV internal diameter at the systolic phase (LVIDs), posterior wall thickness at the diastolic phase (LVPW), and the ejection fraction (EF%) were measured by the Cardiac Measurements Package of Vevo 770 High-Resolution Imaging System.
Chemicals
Chemicals were purchased from Sigma-Aldrich, Inc (St Louis, MO, USA). Verapamil was dissolved in deionized water (dH2O) to create a stock solution.
Statistical analysis
Data are presented as the mean±SEM. The results were analyzed using one-way ANOVA followed by Bonferroni post hoc test for multi-group comparisons. P<0.05 was considered statistically significant.
Results
The expression of CaMKIIδA in CHF and hypoxic cardiomyocytes
A total of 30 rats were randomly assigned to two groups of animals: 1) the sham-operated control rats (n=14); 2) rats with chronic heart failure (CHF, n=16). A representative echocardiography is shown in Figure 1. The ejection fraction (EF) was significantly decreased in the CHF group compared with that in the sham group (P<0.05) (Table 1). Left ventricular internal diameter at the diastolic phase (LVIDd) was increased, and the fractional shortening (FS) was decreased in the CHF rats compared to those in the sham group (Table 1). Left ventricular end diastolic dimension pressure (LVEDP) was significantly increased in the CHF groups compared with that in the sham group (P<0.005). The dp/dt was significantly decreased in the CHF group compared with that in the sham group (P<0.05). Taken together, the increased LVIDd and LVEDP and decreased dp/dt, FS and EF were indicative of cardiac dysfunction in the CHF group. To investigate the alternative splicing of CaMKIIδ in CHF, RNA was extracted and subjected to RT-PCR for the measurement of CaMKIIδ in the left ventricle 4 weeks after LAD artery ligation. We observed CaMKIIδA at the transcription level in the left ventricle of CHF rats (Figure 2A) and in hypoxic neonatal cardiomyocytes (Figure 2B). CaMKIIδA expression was upregulated in the LV of CHF rats compared to that in the LV of the sham controls (P<0.05). CaMKIIδA expression was increased in hypoxic neonatal cardiomyocytes compared to that in controls (P<0.05). The efficiency of the CaMKIIδ siRNA was determined by RT-PCR analysis. The CaMKIIδA siRNAs significantly (P<0.05 vs control) decreased the CaMKIIδA mRNA level by ∼65% (Figure 2C). CaMKIIδA siRNAs did not change the CaMKIIδB or CaMKIIδC mRNA level in control cells (Figure 2C) (P<0.05 vs control).
Echocardiographic changes associated with CHF. Echocardiography was performed 4 weeks after sham surgery (control) or ligation of the left anterior descending coronary artery. The representative M-mode echocardiograms were recorded in sham control (up) and CHF animals (bottom). CHF: chronic heart failure; LVPW: left ventricle posterior wall; LV: left ventricle; IVS: interventricular septal.
Expression of CaMKIIδA in CHF and hypoxic cardiomyocytes. CaMKIIδA mRNA was detected by using RT-PCR in the isolated LV of the heart and neonatal cardiomyocytes. CaMKIIδA mRNA expression was upregulated in CHF animals (A, * P<0.05 vs the sham group) and cardiomyocytes with hypoxia (B). CaMKIIδA siRNA transfection significantly attenuated the increase in hypoxic cardiomyocytes (* P<0.05 vs control; # P<0.05 vs the hypoxia group). CaMKIIδ siRNA efficiency was determined by RT-PCR analysis. CaMKIIδA siRNAs significantly decreased CaMKIIδA mRNA expression (* P<0.05 vs control). CaMKIIδA siRNA did not change CaMKIIδB or CaMKIIδC mRNA expression in control cardiomyocytes (C).
The expression of phosphorylated RyR2 (P-RyR2) in CHF and hypoxic cardiomyocytes
P-RyR2 protein levels were significantly (n=6, P<0.05) upregulated in the left ventricle of CHF rats compared to those in the sham controls (n=6) (Figure 3A). These data indicated that P-RyR2 was activated in rats with CHF. To determine the role of CaMKIIδA in mediating the expression of P-RyR2, CaMKIIδA siRNA was transfected into cultured neonatal cardiomyocytes treated with hypoxia. The expression of P-RyR2 was significantly higher (n=3 cultures, P<0.05) in hypoxic cardiomyocytes than in the control group. CaMKIIδA siRNA significantly (n=3 cultures, P<0.05) normalized the increase in the expression of P-RyR2 in the cardiomyocytes treated with hypoxia (Figure 3B). CaMKIIδA siRNA did not significantly change the expression of P-RyR2 in control cardiomyocytes (Figure 3B). These data indicated that activation of CaMKIIδA in cardiomyocytes in CHF contributed to the upregulation of P-RyR2 expression in the sarcoplasmic reticulum (SR).
Effect of CaMKIIδA siRNA on the expression of phosphorylated ryanodine receptor type 2 (P-RyR2). P-RyR2 protein was detected in the LV of the heart in vivo and in neonatal cardiomyocytes in vitro. (A) P-RyR2 was upregulated in the LV of the heart in the CHF group. * P<0.05 vs the sham group. (B) P-RyR2 protein expression was increased in hypoxic cardiomyocytes compared to that in control cardiomyocytes. CaMKIIδA siRNA transfection significantly reduced the increase in the expression of P-RyR2 in cardiomyocytes. CaMKIIδA siRNA transfection did not significantly change the expression of P-RyR2 in control cardiomyocytes. * P<0.05 vs control cardiomyocytes; # P<0.05 vs cardiomyocytes with hypoxia treatment.
Effect of CaMKIIδA siRNA on SR Ca2+ leak in cardiomyocytes with hypoxia
We then determined the role of CaMKIIδA in the regulation of the SR Ca2+ leak in cardiomyocytes. The present study found that the SR Ca2+ leak in cells with hypoxic treatment (n=12 cells in 3 cultures) was significantly greater than that in control cells (n=11 cells in 3 cultures) (P<0.05, Figure 4A, 4B). CaMKIIδA siRNA significantly (n=16 cells in 3 cultures) normalized the SR Ca2+ leak in cardiomyocytes (P<0.05, Figure 4A, 4B). CaMKIIδA siRNA did not significantly affect the SR Ca2+ leak in control cardiomyocytes (Figure 4A, 4B). These data indicated that upregulation of CaMKIIδA in cardiomyocytes may increase SR Ca2+ leak via upregulation of P-RyR2 expression, potentially contributing to the molecular mechanisms of cardiovascular function impairment in CHF.
Effect of CaMKIIδA siRNA on SR Ca2+ leak. (A) A representative trace of SR Ca2+ leak in the control group, hypoxic cardiomyocytes and CaMKIIδA siRNA-transfected hypoxic cardiomyocytes. (B) SR Ca2+ leak was significantly enhanced in hypoxic cardiomyocytes compared to that in the control group. CaMKIIδA siRNA transfection significantly attenuated the increase in SR Ca2+ leak in hypoxic cardiomyocytes. CaMKIIδA siRNA transfection did not significantly change SR Ca2+ leak in control cardiomyocytes. * P<0.05 vs control cardiomyocytes; # P<0.05 vs cardiomyocytes with hypoxia treatment.
The expression of SERCA2a in CHF and hypoxic cardiomyocytes
SERCA2a protein levels were significantly (n=6, P<0.05) downregulated in the left ventricle of CHF rats compared to the levels in the left ventricle of the sham control animals (n=6) (Figure 5A). These data indicated that SERCA2a was inhibited in rats with CHF. To understand the role of CaMKIIδA in the regulation of SERCA2a in cardiomyocytes, we determined the effect of CaMKIIδA siRNA on SERCA2a expression and tested whether CaMKIIδA siRNA was able to normalize the alteration in SERCA2a expression in hypoxic cardiomyocytes. The present study found that SERCA2a protein levels were significantly decreased by hypoxia treatment in cardiomyocytes (n=3 cultures, P<0.05). This reduction was significantly attenuated in CaMKIIδA siRNA-transfected cells (n=3 cultures, P<0.05) (Figure 5B). CaMKIIδA siRNA did not significantly change the expression of SERCA2a in control cardiomyocytes (Figure 5B). These data indicated that CaMKIIδA upregulation in cardiomyocytes may decrease SR Ca2+ uptake via the downregulation of SERCA2a, potentially contributing to cardiac dysfunction in CHF.
Effect of CaMKIIδA siRNA on SERCA2a expression and activity in cardiomyocytes. SERCA2a protein was detected in the LV of the heart in vivo and in neonatal cardiomyocytes in vitro. (A) SERCA2a protein was detected in the LV of the heart. SERCA2a protein expression was downregulated in the LV of the heart in the CHF group. * P<0.05 vs the sham group. (B) SERCA2a protein was detected in neonatal cardiomyocytes. SERCA2a protein expression was significantly reduced in hypoxic cardiomyocytes compared to that in control cells. CaMKIIδA siRNA transfection significantly attenuated the downregulation in SERCA2a protein expression in hypoxic cardiomyocytes. CaMKIIδA siRNA transfection did not significantly change SERCA2a expression in control cardiomyocytes. * P<0.05 vs control cardiomyocytes; # P<0.05 vs cardiomyocytes with hypoxia treatment.
Discussion
The major findings of this study were as follows: (1) the expression of CaMKIIδA and P-RyR2 was upregulated in the left ventricle of rats with CHF and cardiomyocytes with hypoxia. (2) Downregulation of CaMKIIδA attenuated the activation of P-RyR2 in hypoxic cardiomyocytes. (3) Downregulation of CaMKIIδA reduced SR calcium leak in hypoxic cardiomyocytes. (4) Downregulation of CaMKIIδA normalized the decrease in the expression of SERCA2a in hypoxic cardiomyocytes. These results suggest that inhibition of CaMKIIδA reduces the increase in SR Ca2+ leak elicited by activation of P-RYR2 and reduction in SERCA2a expression in cardiomyocytes with hypoxia.
Upregulation of CaMKII has been suggested to mediate ischemic myocardial injury and cardiac remodeling34. CaMKIIδ is predominantly expressed in the heart and is involved in the process of cardiac hypertrophy and remodeling after pressure overload35. In the present study, we found that CaMKIIδA expression was upregulated in the left ventricle (LV) of CHF animals, consistent with a previous report36. Moreover, the expression of CaMKIIδA was also found to be elevated in ventricular myocytes in rat hypertrophy24. Interestingly, CaMKIIδB was found to be downregulated in abdominal aorta constriction-induced hypertrophy in the rat heart37, and overexpression of CaMKIIδC in transgenic mice has been reported to contribute to the development of cardiac hypertrophy and heart failure38. Therefore, alternative splicing of CaMKIIδ may play an important role in the pathological process of myocardial ischemia.
RyR2 is one of the major downstream targets of CaMKIIδ in cardiac myocytes and plays an important role in regulating Ca2+ release from the SR39. An increased diastolic SR Ca2+ leak is regarded as an important pathological mechanism for the development of cardiac pump failure40. In the present study, the data showed that upregulation of P-RyR2 was not only found in the LV of CHF animals in vivo but also in the hypoxic cardiomyocytes in vitro (Figure 3). In addition, downregulation of CaMKIIδA decreased the Ca2+ leak from the SR in hypoxic cardiomyocytes (Figure 4). These findings are consistent with previous reports showing that inhibition of CaMKII was associated with decreased SR Ca2+ leak41.
Furthermore, our study also revealed that the activation of P-RyR2 induced by hypoxia was effectively blocked by CaMKIIδA siRNA in cardiomyocytes in vitro. These results further suggest that activation of CaMKIIδA induces an increase in SR Ca2+ leak through activation of P-RYR2. The prevention of the increased expression of both CaMKIIδA and P-RyR2 in the cardiomyocytes may contribute to the protective effect on cardiac function.
Activation of RyR2 expressed in the SR of cardiomyocytes contributes to the Ca2+ leak and SERCA2a downregulation in heart failure42,43. Because SERCA2a pumps Ca2+ back to the SR, luminal SR Ca2+ levels are increased and provide the electrochemical driving force for SR Ca2+ release44. Restoration of SERCA2a function might increase spontaneous Ca2+ release and the risk of RyR2-mediated diastolic leak45. In contrast, Lyon AR and colleagues reported that SERCA2a gene therapy can reduce the total SR Ca2+ leak despite the normalized SR Ca2+ load46. Because CaMKIIδA is activated in heart failure, the downregulation of SERCA2a activity in cardiomyocytes may be reasonably thought to be mediated through the upregulation of CaMKIIδA. This speculation was supported by the present study showing that CaMKIIδA siRNA dramatically attenuated the downregulation of SERCA2a expression in neonatal cardiomyocytes induced by hypoxia (Figure 5). Therefore, CaMKIIδA contributed to SR Ca2+ leak through the activation of RyR2 and inhibition of SERCA2a in cardiomyocytes with myocardial ischemia.
CaMKIIδ expression and activity have been demonstrated to be increased in CHF, potentially contributing to cardiac hypertrophy and further worsening CHF47. Whether activation of CaMKIIδA is involved in myocardial ischemia and cardiac remodeling through the activation of RyR2 and downregulation of SERCA2a is not clear. In the present study, elevation of P-RyR2 and decreased expression of SERCA2a induced by hypoxia was effectively attenuated by CaMKIIδA siRNA. Therefore, the therapeutic effect on CHF might involve the deactivation of P-RyR2 and upregulation of SERCA2a through the downregulation of CaMKIIδA.
Conclusion
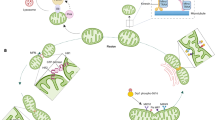
In summary, our study elucidated the potential molecular mechanisms of activation of CaMKIIδA-mediated cardiac dysfunction in CHF (Figure 6). The data from the present study suggested that the inhibition of CaMKIIδA with siRNA may improve cardiac function by preventing SR Ca2+ leak through downregulation of RyR2 and upregulation of SERCA2a expression in cardiomyocytes in CHF. We expect that CaMKIIδA might be a potential target for the prevention and treatment of the cardiac dysfunction associated with CHF in the future.
Schematic representation of our working hypothesis: upregulation of CaMKIIδA enhances the SR Ca2+ leak elicited by the activation of P-RyR2 and the reduction in SERCA2a expression in cardiomyocytes with CHF and hypoxia. CaMKIIδA, calmodulin-dependent kinase II isoform δA; CHF, chronic heart failure; RyR, ryanodine receptor; SERCA2a, Ca2+-ATPase; SR, sarcoplasmic reticulum.
Author contribution
Le GUI, Zhe ZHANG, Xin GUO, Hui XU, and Ya-wei JI performed the experiments; Le GUI, Xin GUO, and Ren-jun WANG analyzed the data; Le GUI, Xin GUO, and Ren-jun WANG prepared the figures; Le GUI, Ren-jun WANG, and Qing-hui CHEN drafted the manuscript; Le GUI, Ren-jun WANG, Jiang-hua ZHU, and Qing-hui CHEN interpreted the results of the experiments; Le GUI, Ren-jun WANG, Jiang-hua ZHU, and Qing-hui CHEN edited and revised the manuscript; Le GUI, Ren-jun WANG, Jiang-hua ZHU, and Qing-hui CHEN approved manuscript.
We thank Jessica BEHNKE for English proof reading and editing.
References
Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 1983; 245: C1–14.
Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol 1994; 126: 839–52.
Gray CB, Heller Brown J. CaMKIIdelta subtypes: localization and function. Front Pharmacol 2014; 5: 15.
Yao J, Qin X, Zhu J, Sheng H. Dyrk1A-ASF-CaMKIIdelta signaling Is involved in valsartan inhibition of cardiac hypertrophy in renovascular hypertensive rats. Cardiology 2016; 133: 198–204.
Schworer CM, Rothblum LI, Thekkumkara TJ, Singer HA. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. J Biol Chem 1993; 268: 14443–9.
Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, et al. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013; 128: 1748–57.
Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, et al. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res 2010; 106: 354–62.
Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 2003; 92: 904–11.
Polakova E, Illaste A, Niggli E, Sobie EA. Maximal acceleration of Ca2+ release refractoriness by beta-adrenergic stimulation requires dual activation of kinases PKA and CaMKII in mouse ventricular myocytes. J Physiol 2015; 583: 1495–507.
Stokke MK, Tovsrud N, Louch WE, Oyehaug L, Hougen K, Sejersted OM, et al. I CaL inhibition prevents arrhythmogenic Ca2+ waves caused by abnormal Ca2+ sensitivity of RyR or SR Ca2+ accumulation. Cardiovasc Res 2013; 98: 315–25.
Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest 2013; 123: 46–52.
Netticadan T, Temsah RM, Kawabata K, Dhalla NS. Sarcoplasmic reticulum Ca2+/calmodulin-dependent protein kinase is altered in heart failure. Circ Res 2000; 86: 596–605.
Bobin P, Varin A, Lefebvre F, Fischmeister R, Vandecasteele G, Leroy J. Calmodulin kinase II inhibition limits the pro-arrhythmic Ca2+ waves induced by cAMP-phosphodiesterase inhibitors. Cardiovasc Res 2016; 110: 151–61.
Woo AY, Xiao RP. beta-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin 2012; 33: 335–41.
Ogrodnik J, Niggli E. Increased Ca2+ leak and spatiotemporal coherence of Ca2+ release in cardiomyocytes during beta-adrenergic stimulation. J Physiol 2010; 588: 225–42.
Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 1993; 72: 463–9.
Yang Y, Rong X, Lv X, Jiang W, Yang Y, Lai D, et al. Inhibition of mevalonate pathway prevents ischemia-induced cardiac dysfunction in rats via RhoA-independent signaling pathway. Cardiovasc Ther 201; 35. doi: 10.1111/1755-5922.12285.
Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 2010; 121: 822–30.
Renaud-Gabardos E, Tatin F, Hantelys F, Lebas B, Calise D, Kunduzova O, et al. Therapeutic benefit and gene network regulation by combined gene transfer of apelin, FGF2, and SERCA2a into ischemic heart. Mol Ther 2017 Nov 16. doi: 10.1016/j.ymthe.2017.11.007
Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 2005; 38: 291–302.
Zhang T, Miyamoto S, Brown JH. Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes? Recent Prog Horm Res 2004; 59: 141–68.
Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, et al. Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ Res 2008; 102: 695–702.
Gray CB, Suetomi T, Xiang S, Mishra S, Blackwood EA, Glembotski CC, et al. CaMKIIdelta subtypes differentially regulate infarct formation following ex vivo myocardial ischemia/reperfusion through NF-kappaB and TNF-alpha. J Mol Cell Cardiol 2017; 103: 48–55.
Li C, Cai X, Sun H, Bai T, Zheng X, Zhou XW, et al. The deltaA isoform of calmodulin kinase II mediates pathological cardiac hypertrophy by interfering with the HDAC4-MEF2 signaling pathway. Biochem Biophys Res Commun 2011; 409: 125–30.
Gui L, Bao Z, Jia Y, Qin X, Cheng ZJ, Zhu J, et al. Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol 2013; 304: H118–130.
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 2006; 114: 1159–68.
Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci 2003; 60: 1376–93.
Chen CC, Kuo CY, Chen RF. Role of CAPE on cardiomyocyte protection via connexin 43 regulation under hypoxia. Int J Med Sci 2016; 13: 754–8.
Pan LJ, Wang X, Ling Y, Gong H. MiR-24 alleviates cardiomyocyte apoptosis after myocardial infarction via targeting BIM. Eur Rev Med Pharmacol Sci 2017; 21: 3088–97.
Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 2002; 91: 594–600.
Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012; 125: 2059–70.
Ji Y, Guo X, Zhang Z, Huang Z, Zhu J, Chen QH, et al. CaMKIIdelta meditates phenylephrine induced cardiomyocyte hypertrophy through store-operated Ca2+ entry. Cardiovasc Pathol 2016; 27: 9–17.
Litwin SE, Katz SE, Morgan JP, Douglas PS. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation 1994; 89: 345–54.
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 2016; 22: 175–82.
Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene 2003; 322: 17–31.
Bell JR, Raaijmakers AJ, Janssens JV, Delbridge LM. CaMKIIdelta and cardiomyocyte Ca2+ signalling new perspectives on splice variant targeting. Clin Exp Pharmacol Physiol 2015; 42: 1327–32.
Liao RJ, Tong LJ, Huang C, Cao WW, Wang YZ, Wang J, et al. Rescue of cardiac failing and remodelling by inhibition of protein phosphatase 1gamma is associated with suppression of the alternative splicing factor-mediated splicing of Ca2+/calmodulin-dependent protein kinase delta. Clin Exp Pharmacol Physiol 2014; 41: 976–85.
Zhang L, Liu BF, Liang S, Jones RL, Lu YT. Molecular and biochemical characterization of a calcium/calmodulin-binding protein kinase from rice. Biochem J 2002; 368: 145–57.
Sondergaard MT, Tian X, Liu Y, Wang R, Chazin WJ, Chen SR, et al. Arrhythmogenic calmodulin mutations affect the activation and termination of cardiac ryanodine receptor-mediated Ca2+ release. J Biol Chem 2015; 290: 26151–62.
Dibb K, Eisner D. A small leak may sink a great ship but what does it do to the heart? J Physiol 2010; 588: 4849.
Tzimas C, Terrovitis J, Lehnart SE, Kranias EG, Sanoudou D. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition ameliorates arrhythmias elicited by junctin ablation under stress conditions. Heart Rhythm 2015; 12: 1599–610.
Respress JL, Gershovich PM, Wang T, Reynolds JO, Skapura DG, Sutton JP, et al. Long-term simulated microgravity causes cardiac RyR2 phosphorylation and arrhythmias in mice. Int J Cardiol 2014; 176: 994–1000.
Hayward C, Banner NR, Morley-Smith A, Lyon AR, Harding SE. The current and future landscape of serca gene therapy for heart failure: a clinical perspective. Hum Gene Ther 2015; 26: 293–304.
Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol 2000; 522 Pt 2: 259–70.
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000; 101: 365–76.
Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 2011; 4: 362–72.
Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 2004; 63: 476–86.
Acknowledgements
This study was supported by operating grants to Le GUI. from the National Natural Science Foundation of China (No 81570294), to Jiang-hua ZHU. from the National Natural Science Foundation of China (No 81370344) and to Qing-hui CHEN from the National Institute of Health (No HL122952).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gui, L., Guo, X., Zhang, Z. et al. Activation of CaMKIIδA promotes Ca2+ leak from the sarcoplasmic reticulum in cardiomyocytes of chronic heart failure rats. Acta Pharmacol Sin 39, 1604–1612 (2018). https://doi.org/10.1038/aps.2018.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2018.20