Abstract
Aim:
LS-007 is a CDK inhibitor, which exhibits potent antitumor activity against chronic lymphocytic leukemia and ovarian cancer cells. In this study, we further evaluated the antitumor activity of LS-007 alone and in combination with a Bcl-2 inhibitor ABT-199 in acute leukemia (AL) cells.
Methods:
Cell viability was detected using resazurin assay, and cell apoptosis was examined using Annexin V/PI double staining and flow cytometry. The inhibition of LS-007 on kinases was evaluated with the mobility shift assay or ELISA. The expression of relevant signaling molecules was assessed using Western blotting and RT-PCR. Primary lymphocytes from patients with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) were separated using Ficoll-Paque PLUS.
Results:
LS-007 inhibited the proliferation of 6 AL cell lines with IC50 values of 100–200 nmol/L, and decreased the survival of ALL and AML patient-derived lymphocytes with mean LD50 value of 67 and 102 nmol/L, respectively. In kinase assays in vitro, LS-007 was more selective for the CDK family, inhibiting CDK2, CDK9, CDK1 and CDK4 at low nanomolar concentrations. In HL-60 and CCRF-CEM cells, LS-007 (0.1–0.4 μmol/L) dose-dependently induced cell apoptosis predominantly through CDK9 inhibition-related dephosphorylation at the ser2 residue of RNA pol II and the corresponding depletion of anti-apoptotic proteins, especially Mcl-1 and XIAP. LS-007 (0.2 and 0.4 μmol/L) also induced cell apoptosis in the patient-derived lymphocytes. In HL-60, CCRF-CEM and Molt-4 cells, combined application of LS-007 with ABT-199 (1 or 2 μmol/L) markedly increased cell apoptosis with a maximal decrease in the XIAP levels as compared with either drug used alone.
Conclusion:
CDK inhibitor LS-007 potently inhibits the established human AL cell lines and primary AL blasts, and it also shows remarkable synergy with Bcl-2 inhibitor ABT-199.
Similar content being viewed by others
Introduction
Acute leukemia (AL) occurs when primitive or immature white blood cells grow uncontrollably, disturbing normal hematopoiesis. According to the pathogenic cellular origin, AL can be divided into acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). ALL is the most common pediatric cancer type1, whereas AML is more prevalent among adults2,3. AL accounts for nearly 27 000 diagnoses and 12 000 deaths yearly in the US4. It progresses quickly and needs immediate treatment. With the progress and availability of combination chemotherapy and hematopoietic cell transplantation, the complete response (CR) rate and overall cure rate of AL patients have improved markedly5. However, ∼20% of ALL patients still experience relapse, and only a minority of them will survive6; more than 60% of AML patients cannot be cured7. Consequently, there is still an urgent need to seek more effective therapy.
Cyclin-dependent kinases (CDKs) are a family of serine/threonine protein kinases whose activity depends on binding to specific cyclin partners8. Some CDKs (CDK1, CDK2, CDK4 and CDK6) have a role in regulating cell cycle transition9. Another family of CDKs that regulates transcription was also identified and includes CDK7 and CDK98,10. CDK7 is an integral part of transcription factor II H (TFIIH), which phosphorylates the serine 5 (ser5) residue of RNA polymerase II (RNA pol II) to facilitate transcription initiation11. CDK9, as the catalytic part of elongation factor P-TEFb, phosphorylates the serine 2 (ser2) residue of RNA pol II, which is required for efficient transcription elongation11. Owing to the central role of CDKs in the control of cell division, it is not surprising that cancers exhibit some features that derange the normal controls over the cell cycle. Additionally, over the past 20 years, numerous drugs that target cell cycle-related CDKs have emerged and have been tested in clinical trials12. Recent studies have indicated that the inhibition of transcription-related CDK9 might contribute predominantly to the anti-tumor activity of most clinical CDK inhibitors, such as flavopiridol and dinaciclib13,14,15. CDK9 is essential for the efficient transcription of numerous genes, especially those encoding short-lived anti-apoptotic proteins, such as Mcl-1, XIAP and Bcl-213,16. Additionally, CDK9 inhibition through siRNA or inhibitors induces potent cell apoptosis in many tumor cell lines17,18.
LS-007, also known as CDKI-73, is one of the most potent CDK9 inhibitors identified to date19. Previous studies have shown that LS-007 had little toxicity on normal T- and B-cells while exhibiting potent efficiency alone or in combination with fludarabine against chronic lymphocytic leukemia (CLL) primary cells19. In addition, LS-007 exerted a potent anti-tumor effect in ovarian cancer A2780 cell lines through simultaneously targeting CDK9 and its downstream Mnk-eIF4E pathways20. These results provided convincing evidence that LS-007 may be a promising anti-tumor candidate deserving further development. CDK9 is often abnormally activated in lymphoid or myeloid leukemia, especially in those with re-arranged mixed lineage leukemia21,22. Therefore, in this study, we evaluated the effects of LS-007 in AL cell lines and primary AL blasts both as a single agent and in combination with the BH3 mimetic ABT-199. Our data demonstrated that LS-007 exhibited activity as a single agent and exhibited a remarkable degree of synergy with ABT-199 in AL.
Materials and methods
Compounds
LS-007 was kindly provided by Prof Shu-dong WANG (University of South Australia, Australia). Flavopiridol HCl (S2679) and ABT-199 (S8048) were purchased from Selleck (Houston, TX, USA). The compounds were dissolved in DMSO at 10 mmol/L and stored at −80 °C.
In vitro kinase assay
The inhibition of CDKs was measured using mobility shift assays by ShangHai ChemPartner (Shanghai, China). The inhibition of other kinases was measured by WuXi AppTec (Shanghai, China) or by enzyme immunosorbent assays23.
Cell culture
The human acute leukemia cell lines CCRF-CEM, U937, and Molt-4 were purchased from the American Type Culture Collection (Manassas, VA, USA). The HL-60 and THP-1 cell lines were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). Jurkat cells were kindly gifted by Prof Jian-ping ZUO (SIMM, Shanghai, China). All of the cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 4.5 g/L glucose, 0.11 g/L sodium pyruvate, and 10% FBS (Gibco, Grand Island, NY, USA) and maintained at 37 °C and in 5% CO2.
Proliferation assays
Cells were seeded at the appropriate density in 96-well plates and cultured for 2 h before exposure to increasing doses of LS-007 or flavopiridol. After 72 h of incubation, 10% 1.5 mg/mL resazurin (Sigma, St Louis, MO, USA) was added, and the cells were incubated for another 2–4 h. Fluorescence intensity was detected at an excitation wavelength range of 540±35 nm and an emission wavelength range of 590±35 nm using a Synergy 2 Multi-Mode Microplate Reader (BioTek, Burlington, VT, USA). The dosages corresponding to the half-maximal inhibition (IC50) were calculated using SoftMax Pro-based non-liner 4-parameter regression analysis. IC50 (mean±SD) values are presented as histograms.
Apoptosis assays
An Annexin V-FITC/PI double staining apoptosis detection kit (KeyGEN Biotech, Nanjing, China) was used to quantitatively measure the percentage of cells in apoptosis. Cells (5×105 per well in 6-well plates) were incubated in media alone or with various concentrations of LS-007 (or flavopiridol) for 24 h or were incubated with 0.2 μmol/L of the test compounds for 12, 24, or 48 h. The samples were collected, stained, and detected using a FACS Calibur instrument (BD Biosciences, Franklin Lake, NJ, USA). The results were analyzed using BD CellQuest Pro software.
Western blotting
Cells were cultured for 2 or 24 h with various concentrations of LS-007 (or flavopiridol), harvested, washed and lysed with RIPA lysis buffer (strong) (Beyotime, Haimen, China). The protein concentration of lysates was unified, mixed with gel electrophoresis loading buffer, and boiled for 5–10 min. Subsequently, the cell lysates were resolved using SDS–PAGE, were transferred to nitrocellulose membranes and then were probed using the following primary antibodies: RNA pol II, p-RNA pol II ser2, p-RNA pol II ser5 (Abcam, Cambridge, Cambridgeshire, UK); RB, p-RB ser780, p-RB ser807/811, PP1α, p-PP1α thr320, β-tubulin, PARP, XIAP, Mcl-1, Bcl-2, cleaved-caspase3 (Cell Signaling, Boston, MA, USA); p-RB thr821 (Gene Tex, San Antonio, TX, USA). Next, the membranes were incubated with anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase, and the proteins were detected using an enhanced chemiluminescence detection reagent (Thermo Fisher Scientific, Rockford, IL, USA) and ImageQuant LAS 4000 (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Real-time PCR
Cells were left untreated or were exposed to LS-007 (or flavopiridol) for 6 h. Total cellular RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into complementary DNA using a PrimeScript™ RT reagent kit (Takara, Otsu, Shiga, Japan). Real-time PCR was then performed using Applied Biosystems ViiATM7 (Waltham, MA, USA) and SYBR-Green Master mix (Takara, Otsu, Shiga, Japan) using the following primers (Table 1).
The reaction parameters were as follows: 95 °C for 5 min followed by 41 cycles of 95 °C for 5 s and 60 °C for 34 s. All samples, including the controls, were tested in duplicate.
Primary lymphocyte isolation
Bone marrow samples from ALL and AML pediatric patients were obtained according to the provision of informed consent using the guidelines approved by the Committee on the Use of Human Subjects in Research at Shanghai Children's Hospital and the Children's Hospital of Fudan University. Mononuclear cells were separated from freshly collected bone marrow using Ficoll-Plaque Plus (GE Healthcare, Little Chalfont, Buckinghamshire, UK) density gradient centrifugation following the manufacturer's instructions. Subsequently, enriched cells were maintained in RPMI-1640 medium supplemented with 10% FBS and were immediately seeded in 96-well plates for viability tests after 72 h of treatment with LS-007/flavopiridol. Additionally, the cells were seeded in 12-well plates and exposed to LS-007/flavopiridol for 48 h (for apoptosis detectuion) or 12 h (for protein expression detection).
Statistical analysis
Combination index (CI) values were calculated using the IC50 ratio (50% of cells that underwent apoptosis at that dose) obtained with LS-007 alone to that obtained with LS-007 plus ABT-199 as previously described24. Student's t test was applied for statistical comparison using GraphPad Prism 6. Unless otherwise indicated, the results are expressed as the mean±SD from at least three independent experiments. Differences were considered to have statistically significant at P<0.05, P<0.01 vs CON; P<0.01 vs ABT-199 and P<0.01 vs LS-007.
Results
LS-007 potently inhibits the proliferation of AL cell lines and patient-derived AL blasts
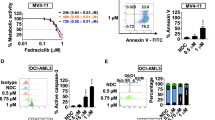
The activity of LS-007 against human acute leukemia was evaluated in a panel of established AL cell lines, including ALL and AML cell lines. As shown in Figure 1A, LS-007 exhibited potent cytotoxicity against these cell lines, with IC50 values ranging from 100–200 nmol/L, and the mean IC50 for all of the tested leukemia cell lines was 163±48 nmol/L, which was similar to that of flavopiridol (147±25 nmol/L).
LS-007 exhibits potent anti-proliferative activity against acute leukemia cells. (A) Six established human acute leukemia cell lines (data are expressed as the mean±SD from at least three repeat assays) and primary lymphocytes derived from ALL (B) and AML (C) pediatric patients were treated with increasing concentrations of LS-007 or flavopiridol for 72 h.
Next, bone marrow samples from 21 ALL and 15 AML pediatric patients were also tested to obtain results that are more clinically relevant. Lymphocytes were separated using Ficoll-Paque PLUS for corresponding experiments25. Similarly to the effects on cell lines, LS-007 greatly affected the survival of all of these patients-derived lymphocytes, with a mean LD50 value of 67 nmol/L against ALL (Figure 1B) and 102 nmol/L against AML (Figure 1C) cells, values that were slightly lower than those for flavopiridol (LD50 of 100 nmol/L, 112 nmol/L, respectively) (Figure 1B, 1C). It was noteworthy that LS-007 also exhibited cytotoxicity in one of two relapsed ALL samples with an LD50 value of 5 nmol/L, although this result needs to be confirmed.
LS-007 is a potent and selective inhibitor of CDK
Inhibition of the kinase activity of various cyclin/CDKs was examined using a mobility shift assay at the molecular level. Similar to previously reported data19, we also demonstrated that LS-007 was a potent CDK9 inhibitor, with an IC50 value of 5.78 nmol/L. Additionally, LS-007 inhibited CDK2, CDK1 and CDK4 with IC50 values of 3.27, 8.17 and 8.18 nmol/L, respectively. Compared to flavopiridol, LS-007 was an equally potent inhibitor of CDK9 but exhibited 1.8-, 2.6- and 17-fold stronger inhibition of CDK1, CDK4 and CDK2, respectively. LS-007 also inhibited CDK6 and CDK7 (IC50: 37.68 and 134.26 nmol/L, respectively) but was much weaker than the other tested CDKs (Table 2). The efficacy against CDK8 was also measured; only a 28% inhibitory rate was found at 100 nmol/L LS-007 (Table 3).
However, LS-007 is not a general kinase inhibitor and, in a series of 41 additional kinase counter screens, was more selective for the CDK family. Only Gsk3β activity was inhibited by 55% after treatment with 100 nmol/L LS-007 (Table 3). It has been reported that flavopiridol affects a broader range of serine/threonine and tyrosine kinases26, possibly leading to its narrow therapeutic index.
LS-007 inhibits cellular CDK activity
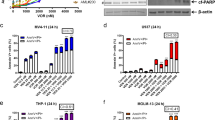
We next investigated the effect of LS-007 on cellular CDK activity in two AL cell lines: HL-60 and CCRF-CEM. As illustrated in Figure 2, ser2 phosphorylation of the carboxy-terminal domain (CTD) of RNA pol II was attenuated upon exposure to 0.1 μmol/L of LS-007 for 2 h and almost completely disappeared at 0.2 μmol/L, indicating remarkable cellular CDK9 inhibition. Moreover, compared with control-treated cells, LS-007 also attenuated the phosphorylation of PP1α (thr320) and RNA pol II CTD (ser5) in a concentration-dependent manner (Figure 2A). There was slightly greater inhibition of ser2 phosphorylation than that of ser5 and PP1α, consistent with a lower IC50 of CDK9. However, we also observed that this compound elicited no effect on the phosphorylation of RB at concentrations within the 0.1–0.4 μmol/L range, indicating that LS-007 negligibly impairs CDK2/CDK4/CDK6 activity at concentrations less than 0.4 μmol/L at the cellular level (Figure 2). Significant suppression of phosphorylation of RNA pol II (ser2) was also found in primary cells treated with LS-007, indicating that LS-007 potently attenuated cellular CDK9 activity in primary cells (Figure 3F).
LS-007 inhibits CDK1/CDK7/CDK9 activity in AL cells. HL-60 (A), CCRF-CEM (B) cells were treated with increasing concentrations of LS-007 or flavopiridol for 2 h, and cell lysates were collected and examined by immunoblotting with the indicated antibodies.
LS-007 induces apoptosis in a dose- and time-dependent manner. CCRF-CEM and HL-60 cells were treated with increasing concentrations of LS-007 for 24 h (A) or with 0.2 mol/L LS-007 for 12, 24, or 48 h (B), and 5 patient-derived lymphocytes were treated with LS-007 for 48 h (C). Apoptotic cells were detected by Annexin V+/PI staining and analyzed by flow cytometry. Cells (D, E) or patient-derived lymphocytes (F) were treated with LS-007 or flavopiridol and then were collected for Western blotting (D, F) and qPCR for Mcl-1 or XIAP (E). *P<0.05, **P<0.01 vs CON.
LS-007 induces dose- and time-dependent apoptosis in established AL cell lines and patient-derived AL blasts
The mechanism of LS-007-induced cell killing was confirmed to be through apoptosis (Figure 3A–3C),which was mediated in a concentration- and time-dependent manner, as indicated by flow cytometric analysis using Annexin V/PI double staining. After treatment with 0.1 μmol/L LS-007 for 24 h, approximately 20% of HL-60 cells underwent apoptosis. Additionally, 80% of cells with well-characterized apoptosis were observed at the concentration of 0.4 μmol/L (Figure 3A). Treatment with 0.2 μmol/L LS-007 significantly increased apoptotic cells compared with control cells within 12 h, and this population continued to increase over time (Figure 3B). The apoptotic cleavage of poly (ADP-ribose) polymerase (PARP) and caspase 3 were also increased in a dose-dependent manner by LS-007 (Figure 3D, 3F). In addition, increased numbers of apoptotic cells were observed in 5 tested primary leukemia samples that were exposed to 0.2 or 0.4 μmol/L LS-007 for 48 h (Figure 3C). Collectively, these findings suggest that LS-007 induces apoptosis in AL cells.
LS-007 down-regulates both the transcriptional and protein levels of XIAP and Mcl-1
Inhibition of transcriptional CDKs may induce cell death through the down-regulation of several short-lived proteins, including the anti-apoptotic proteins Mcl-1 and XIAP24. We therefore examined the change in Mcl-1, XIAP and Bcl-2. Indeed, the expression of Mcl-1 and XIAP was dose-dependently decreased after treatment with LS-007 (Figure 3D, 3F), and this effect was mediated by significant inhibition at the transcription level (Figure 3E). However, no reduction in the Bcl-2 protein level was observed following exposure to LS-007. The above results re-emphasized that LS-007 inhibits CDK9 activity and is efficient in both cultured acute leukemia cell lines and primary leukemia lymphocytes.
The combination of LS-007 and ABT-199 synergistically induces AL cell apoptosis
ABT-199 (venetoclax) is currently being evaluated in clinical trials for the treatment of leukemia. It is a highly potent, orally bioavailable Bcl-2-selective inhibitor and shows promise for the treatment of Bcl-2-dependent hematological malignancies27,28,29,30,31. Therefore, we explored whether there is a synergistic interaction between LS-007 and ABT-199 against AL cells. Three human AL cell lines, HL-60, CCRF-CEM and Molt-4, were treated with increasing doses of LS-007 alone or concurrently with ABT-199. The concentration of ABT-199 was determined from preliminary experiments. At the indicated concentration of ABT-199 alone, compared with the control, a 10%–20% inhibitory rate was achieved in HL-60, Molt-4 and CCRF-CEM cells. The combination of LS-007 and ABT-199 produced marked synergistic apoptosis induction against these cell lines, demonstrated by an obvious left shift of the alive-dose curves. We also determined the combination therapy effect by calculating the combination index (CI). CI values were calculated by the ratio of concentrations inducing 50% cellular apoptosis obtained with LS-007 combined with ABT-199 to the ratio obtained with LS-007 alone as described previously24. CI values less than 0.8 indicates synergism32. The mean CI values from three independent experiments were 0.44±0.03, 0.61±0.17 and 0.54±0.13 for HL-60, Molt-4 and CCRF-CEM cells, respectively (Figure 4A–4C).
The combination of LS-007 (L) and ABT-199 (A) synergistically induces cell apoptosis in AL cells. HL-60 (A), Molt-4 (B) and CCRF-CEM (C) cells were treated with different doses of LS-007 alone or in the presence of ABT-199 at 1 μmol/L (HL-60, Molt-4) or 2 μmol/L (CCRF-CEM) for 24 h. Apoptosis was further detected at a fixed concentration of 0.1 μmol/L LS-007 alone or in the presence of ABT-199 at 1 μmol/L (HL-60, Molt-4) or 2 μmol/L (CCRF-CEM) for 24 h. Apoptosis was further measured by FACS analysis (D), and apoptosis-related proteins were detected by Western blotting (E). **P<0.01 vs CON; ##P<0.01 vs ABT-199 and &&P<0.01 vs LS-007.
The Annexin V/PI assay further revealed that LS-007 or ABT-199 alone weakly increased the accumulation of apoptosis in vitro. However, combined therapy resulted in a dramatic increase in apoptotic cells (Figure 4D). Consistent with this result, increased cleaved PARP and caspase 3 were also observed (Figure 4E). Next, the effects of combination therapy on apoptosis signaling were assayed. As expected, ABT-199 or LS-007 alone modestly reduced XIAP expression, whereas the maximal suppression of XIAP was observed in dual-treated cells. Up-regulation of Mcl-1 may mediate resistance to Bcl-2 inhibitors in many tumors33. In the present study, ABT-199 alone slightly elevated the level of Mcl-1, and LS-007 reversed the up-regulation in dual-treated cells. However, no alteration in Bcl-2 was observed using the two drugs alone or combined.
Discussion
Given the key role of CDKs in both cell cycle regulation and cellular transcription processes, which are frequently altered in malignant cells, their potential as molecular targets for anti-cancer therapies has become increasingly recognized12. With the development of the pan-CDK inhibitor flavopiridol, more specific CDK inhibitors have been developed with encouraging results12. In this study, we present our findings on the CDK inhibitor LS-007 in acute leukemia both alone and in combination with ABT-199. LS-007 has been reported to be one of the most efficient CDK9 inhibitors and is effective in both CLL and ovarian tumors19,20. We evaluated the inhibitory activity of LS-007 against various CDKs and 41 types of non-CDK kinases at the molecular level. Consistent with previous reports19, LS-007 effectively inhibits the activity of CDK1, 2, and 9. LS-007 also obviously attenuates CDK4 activity while displaying a lower potency against CDK6/7 and most non-CDK kinases in biochemical kinase assays. Compared to flavopiridol, LS-007 appears to be an equally potent inhibitor of CDK9 and more selective for the CDK family. By contrast, flavopiridol can affect the activity of EGFR and some other non-CDK kinases, as well as CDKs26. Furthermore, we investigated the activities downstream of CDK inhibition-related phosphorylation of RNA pol II, PP1α and RB. We showed a concentration-dependent de-phosphorylation of CDK9 downstream involving the RNA pol II CTD at ser2 residues. Moreover, the dose-dependent de-phosphorylation of PP1α at thr320 was also displayed using the same concentrations of LS-007, indicating CDK1 inhibition at the cellular level. In contrast to the biochemical kinase assay results, LS-007 hardly altered the RB phosphorylation status, which is an indicator of CDK2/4/6 activities, in CCRF-CEM and HL-60 cells.
Hematologic malignancies, such as AL, often have a high demand for the transcription and translation of anti-apoptotic proteins to resist apoptosis34. Many of these proteins have short half-lives at both the mRNA and protein levels35. Additionally, the inhibition of transcriptional CDKs might induce cell death through the down-regulation of these short-lived anti-apoptotic proteins, including Mcl-1 and XIAP36. In our study, LS-007 resulted in in vitro cell killing, at a sub-micromolar level, in AL cell lines and in samples derived from 21 ALL and 15 AML pediatric patients accompanied by the induction of apoptosis. As expected, LS-007 and flavopiridol clearly induced Mcl-1 and XIAP down-regulation via transcriptional repression caused by CDK9-related RNA pol II de-phosphorylation. However, the expression of Bcl-2 remained unchanged. These findings are consistent with previous studies of flavopiridol and SNS-03211,14.
CDK inhibitors always show tremendous preclinical activity, but their clinical application was limited by modest efficacy along with various side effects12. Nevertheless, it seems encouraging that the combination of CDK inhibitors with other chemotherapeutic drugs will enhance the efficacy and reduce unfavorable toxicity of CDK inhibitors, which has been confirmed in many preclinical and clinical settings aimed at lowering the cancer burden37,38. On the other side, the over-expression of the anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-xL, and Mcl-1 is frequently associated with acute leukemia progression and resistance to conventional chemotherapies39,40,41. Bcl-2 and Mcl-1 usually compensate each other's function in tumor cells42. Our previous data demonstrated that LS-007 had little effect on the Bcl-2 levels, although it could significantly reduce Mcl-1 expression. Thus, we hypothesized that Bcl-2 inhibitors may have the potential to be effective in combination with LS-007 toward a broad spectrum of hematological malignancies. ABT-199 is a highly selective Bcl-2 inhibitor and was granted breakthrough designation by the FDA for relapsed or refractory CLL with a 17p deletion28,40. In addition, ABT-199 showed potent efficacy in treating AL, especially AML27 and ALL29, and a phase II trial in subjects with AML has already been completed40. Therefore, here, we used this compound for the investigation of the synergistic effect. As expected, apoptosis induced by co-treatment with LS-007 and ABT-199 was remarkable without reaching the dosages inducing obvious apoptosis by LS-007 or ABT-199 alone, indicating stronger efficacy with less toxicity at the same time. In contrast to a previous study, in which the CDK9 inhibitor wogonin potentiated the anti-cancer activity of the Bcl-2 family inhibitor ABT-263 mainly through down-regulating the Mcl-1 levels33, maximal suppression of XIAP, other than through Mcl-1, was observed when LS-007 was combined with ABT-199. As an anti-apoptotic protein, the synergistic inhibition of XIAP may contribute to the greatly increased cells in apoptosis. Moreover, LS-007 inhibited the phosphorylation of RNA pol II, induced apoptosis in leukemia associated with the down-regulation of Mcl-1, whose up-regulation was thought to confer drug resistance when exposed to Bcl-2 inhibitors, and might delay drug resistance caused by Bcl-2 inhibitors. However, the actual mechanism of such a combination needs to be further explored.
Altogether, our study demonstrated that LS-007, a novel CDK inhibitor, showed potent anti-tumor activity against AL cell lines and primary AL blasts and provides a rational for including AML and ALL patients in clinical trials for LS-007 in combination with ABT-199.
Author contribution
Shao XIE, Yi CHEN and Jian DING designed the experiments; Shu-dong WANG provided the LS-007 compound; Shao XIE, Hui JIANG, Xiao-wen ZHAI, and Fan WEI performed the research; Shao XIE and Yi CHEN analyzed the data and wrote the paper, which was revised by Jian DING and Yi CHEN.
References
Hiroto I, Mel G, Mullighan CG . Acute lymphoblastic leukemia. Lancet 2013; 381: 198–251.
Deschler B, Lubbert M . Acute myeloid leukemia: epidemiology and etiology. Cancer 2006; 107: 2099–107.
Xiong XX, Liu JM, Qiu XY, Pan F, Yu SB, Chen XQ . Piperlongumine induces apoptotic and autophagic death of the primary myeloid leukemia cells from patients via activation of ROS-p38/JNK pathways. Acta Pharmacol Sin 2015; 36: 362–74.
Siegel R, Miller K, Jemal A . Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29.
Soulier J, Cortes J . Introduction to the review series on acute lymphoblastic leukemia. Blood 2015; 125: 3965–6.
Muschen M . Rationale for targeting the pre-B-cell receptor signaling pathway in acute lymphoblastic leukemia. Blood 2015; 125: 3688–93.
Döhner H, Weisdorf DJ, Bloomfield CD . Acute myeloid leukemia. N Engl J Med 2015; 373: 1136–52.
Malumbres M . Cyclin-dependent kinases. Genome Biol 2014; 15: 122.
Malumbres M, Barbacid M . Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153–66.
Lim S, Kaldis P . Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013; 140: 3079–93.
Chen R, Wierda WG, Chubb S, Hawtin RE, Fox JA, Keating MJ, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009; 113: 4637–45.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES . The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Disc 2015; 14: 130–46.
Wang S, Fischer PM . Cyclin-dependent kinase 9: a key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol Sci 2008; 29: 302–13.
Chen R, Keating MJ, Gandhi V, Plunkett W . Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood 2005; 106: 2513–9.
Gregory GP, Hogg SJ, Kats LM, Vidacs E, Baker AJ, Gilan O, et al. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia 2015; 29: 1437–41.
Krystof V, Baumli S, Furst R . Perspective of cyclin-dependent kinase 9 (CDK9) as a drug target. Curr Pharm Des 2012; 18: 2883–90.
Cai D . Latham VM Jr, Zhang X, Shapiro GI . Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res 2006; 66: 9270–80.
Xie G, Tang H, Wu S, Chen J, Liu J, Liao C . The cyclin-dependent kinase inhibitor SNS-032 induces apoptosis in breast cancer cells via depletion of Mcl-1 and X-linked inhibitor of apoptosis protein and displays anti-tumor activity in vivo. Int J Oncol 2014; 45: 804–12.
Walsby E, Pratt G, Shao H, Abbas AY, Fischer PM, Bradshaw TD, et al. A novel Cdk9 inhibitor preferentially targets tumor cells and synergizes with fludarabine. Oncotarget 2014; 5: 375–85.
Lam F, Abbas AY, Shao H, Teo T, Adams J, Li P, et al. Targeting RNA transcription and translation in ovarian cancer cells with pharmacological inhibitor CDKI-73. Oncotarget 2014; 5: 7691–704.
Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell 2010; 37: 429–37.
Frame S, Hogben M, Munro C, Blake DG, Green SR, Zheleva DI . Abstract 3886: Therapeutic potential of CDK inhibitors in MLL leukemias. Cancer Res 2010; 70.
Peng T, Wu JR, Tong LJ, Li MY, Chen F, Leng YX, et al. Identification of DW532 as a novel anti-tumor agent targeting both kinases and tubulin. Acta Pharmacol Sin 2014; 35: 916–28.
Li X, Tong LJ, Ding J, Meng LH . Systematic combination screening reveals synergism between rapamycin and sunitinib against human lung cancer. Cancer Lett 2014; 342: 159–66.
Lehner M, Holter W . Endotoxin-free purification of monocytes for dendritic cell generation via discontinuous density gradient centrifugation based on diluted Ficoll-Paque Plus. Int Arch Allergy Immunol 2002; 128: 73–6.
Sedlacek HH . Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol 2001; 38: 139–70.
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 2014; 4: 362–75.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves anti-tumor activity while sparing platelets. Nat Med 2013; 19: 202–8.
Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood 2014; 124: 3738–47.
Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 2014; 28: 210–2.
Vogler M, Dinsdale D, Dyer MJ, Cohen GM . ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br J Haematol 2013; 163: 139–42.
Bijnsdorp IV, Giovannetti E, Peters GJ . Analysis of drug interactions. Methods Mol Biol 2011; 731: 421–34.
Polier G, Giaisi M, Kohler R, Muller WW, Lutz C, Buss EC, et al. Targeting CDK9 by wogonin and related natural flavones potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263. Int J Cancer 2015; 136: 688–98.
Bose P, Simmons GL, Grant S . Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs 2013; 22: 723–38.
Koumenis C, Giaccia A . Transformed cells require continuous activity of RNA polymerase II to resist oncogene-induced apoptosis. Mol Cell Biol 1997; 17: 7306–16.
Shapiro GI . Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 2006; 24: 1770–83.
Deep G, Agarwal R . New combination therapies with cell-cycle agents. Curr Opin Investig Drugs 2008; 9: 591–604.
Malumbres M, Pevarello P, Barbacid M, Bischoff JR . CDK inhibitors in cancer therapy: what is next? Trends Pharmacol Sci 2008; 29: 16–21.
Bose P, Grant S . Mcl-1 as a therapeutic target in acute myelogenous leukemia (AML). Leuk Res Rep 2013; 2: 12–4.
Cang S, Iragavarapu C, Savooji J, Song Y, Liu D . ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol 2015; 8: 129.
Su LY, Shi YX, Yan MR, Xi Y, Su XL . Anticancer bioactive peptides suppress human colorectal tumor cell growth and induce apoptosis via modulating the PARP-p53-Mcl-1 signaling pathway. Acta Pharmacol Sin 2015; 36: 1514–9.
Li L, Pongtornpipat P, Tiutan T, Kendrick SL, Park S, Persky DO, et al. Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia 2015; 29: 1702–12.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (81521005) and the National Basic Research Program of China (2013CB932503).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, S., Jiang, H., Zhai, Xw. et al. Antitumor action of CDK inhibitor LS-007 as a single agent and in combination with ABT-199 against human acute leukemia cells. Acta Pharmacol Sin 37, 1481–1489 (2016). https://doi.org/10.1038/aps.2016.49
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.49
Keywords
This article is cited by
-
A compound combination screening approach with potential to identify new treatment options for paediatric acute myeloid leukaemia
Scientific Reports (2020)
-
CDK9 inhibitors in acute myeloid leukemia
Journal of Experimental & Clinical Cancer Research (2018)
-
Repression of Mcl-1 expression by the CDC7/CDK9 inhibitor PHA-767491 overcomes bone marrow stroma-mediated drug resistance in AML
Scientific Reports (2018)
-
Evaluation of in vitro and in vivo activity of a multityrosine kinase inhibitor, AL3810, against human thyroid cancer
Acta Pharmacologica Sinica (2017)
-
Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition
Scientific Reports (2017)