Abstract
Aberrant activation of the PI3K/Akt/mTOR pathway contributes to the proliferation of malignant cells, and may confer resistance to chemotherapy in various malignancies, including acute myeloid leukemia (AML). Chemoresistance is the major reason for relapse in AML. RAD001 (everolimus) has been used at d1 and d7 of an induction chemotherapy regimen for AML, which has acceptable toxicity and may improve conventional chemotherapeutic treatment. Dual inhibitors of PI3K and mTOR overcome some of the intrinsic disadvantages of rapamycin and its derivatives. In this study, we evaluated the effects of BEZ235, a PI3K/mTOR dual inhibitor, on the multidrug-resistant AML cell lines HL-60/VCR and K562/ADR in vitro. BEZ235 dose-dependently inhibited the viability of HL-60/VCR and K562/ADR cells with the IC50 values of 66.69 and 71.44 nmol/L, respectively. BEZ235 (25–100 nmol/L) dose-dependently inhibited the migration of the two AML cell lines, and it also significantly sensitized the two AML cell lines to VCR and ADR. After treatment with BEZ235, the miR-1-3p levels were markedly increased in HL-60/VCR cells. Using TargetScan analysis and luciferase assays, we showed that miR-1-3p targeted BAG4, EDN1 and ABCB1, the key regulators of cell apoptosis, migration and multidrug resistance, and significantly decreased their levels in the two AML cell lines. Transfection of HL-60/VCR and K562/ADR cells with miR-1-3p-AMO to inhibit miR-1-3p could reverse the anti-proliferation effects of BEZ235. In conclusion, the PI3K/mTOR dual inhibitor BEZ235 effectively chemosensitizes AML cells via increasing miR-1-3p and subsequently down-regulating BAG4, EDN1 and ABCB1.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is the most common hematological malignancy, and the incidence is increasing as the population ages. AML consists of biologically complex and molecularly and clinically diverse subtypes. New molecular technologies have allowed for in-depth molecular analyses of AML patients using risk-adapted stratification approaches. More and more novel mutations, epigenetic changes, and/or aberrant expression levels of protein-coding and noncoding genes have been shown to be involved in leukemogenesis1,2. Despite advances in understanding the etiology and pathogenesis of AML, the treatment of refractory or relapsed AML (rrAML) remains a daunting clinical challenge. One of the most important reasons is multiple drug resistance. Although hematological malignancies are generally responsive to chemotherapy, and high remission rates are obtained, disease-related death is the rule rather than the exception. Approximately 15%–30% of AML patients are primarily resistant to chemotherapy, and 60%–80% of patients who achieve complete remission will inevitably relapse and succumb to the disease3,4. The PI3K/AKT/mTOR pathway has a crucial role in various normal and abnormal biological and physiological processes, including metabolism, migration, survival, autophagy, lysosome biogenesis and growth transcription, translation, cell-cycle progression, apoptosis and chemotherapy resistance5,6,7,8. Activation of PI3K activates downstream serine/threonine kinases in turn. Phosphorylation of Akt reduces the tuberous sclerosis complexes 1 and 2 (TSC1 and TSC2) but activates the mTOR complex 1 (mTORC1) and subsequent phosphorylation of 70S6K1, S6, and the eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), resulting in increased translation and protein synthesis. Another complex of mTOR, mTORC2, has been identified more recently and appears to act as a feedback loop via Akt. Activation of mTORC2 via phosphorylated 70S6K1 induces phosphorylation of Akt, thereby inhibiting mTORC1 activation. Overactivation of PI3K/Akt/mTOR plays a pivotal role in many human cancers. Previous studies have shown that in approximately 50%–70% of AML cases, the PI3K/Akt/mTOR pathway is constitutively activated9, indicating it could be a potential therapeutic target. Multiple new drugs are being developed to treat AML, including PI3K/AKT/mTOR inhibitors. Park conducted a phase Ib trial assessing the combination of RAD001 (everolimus), an allosteric inhibitor of mTORC1, and conventional chemotherapy in AML patients under 65 years of age at first relapse. They found that a 70 mg dose of RAD001 at d1 and d7 of an induction chemotherapy regimen for AML had acceptable toxicity and may improve treatment efficacy10. BEZ235 is an imidazo[4,5-c]quinoline derivative that inhibits PI3K and mTOR kinase activity by binding to the ATP-binding cleft of these enzymes. BEZ235 has shown beneficial effects on a variety of tumors in vivo and in vitro, including lymphoid malignancies11,12. Moreover, it could reverse chemoresistance13 and overcome radioresistance14. Hall et al reported that BEZ235 modulated glucocorticoid resistance in T-ALL15. However, the effects of the PI3K/mTOR dual inhibitor BEZ235 on AML and the underlying mechanisms are unclear. Here, we analyzed changes in proliferation, apoptosis and migration in AML multidrug-resistant cell lines after treatment with BEZ235. Moreover, we also identified changes in miRs using a miR array and explored the potential proximate mechanisms.
Materials and methods
Cell culture and cell transfection
HL-60/VCR, K562/ADR and HEK-293T cells were stored in our lab. At 37 °C in a 5% humidified atmosphere, HL-60/VCR and K562/ADR cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Inc, Waltham, MA, USA), and HEK 293T cells were cultured in DMEM (Thermo Fisher Scientific, Inc, Waltham, MA, USA). All media contained 10% fetal bovine serum (Thermo Fisher Scientific, Inc, Waltham, MA, USA). After the cells were starved in serum-free RPMI-1640 overnight, cell transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's instructions. Scramble miR (miR-NC), miR-1-3p inhibitor (miR-1-3p-AMO), and Lipofectamine 2000 were diluted with serum-free RPMI-1640. First, the diluted Lipofectamine 2000 was added to the diluted miR and incubated for 20 min at room temperature. Then, they were added to the cell suspension. After culturing for 6 h, the medium was changed to fresh RPMI-1640 with 10% FBS.
Cell proliferation assay
Vincristine (VCR) and adriamycin (ADR) were purchased from Hengrui Medicine Co (Lianyungang, China). BEZ235 was purchased from Selleckchem (USA), dissolved in dimethyl sulfoxide (DMSO) at 10 mmol/L and stored frozen in aliquots. The cells were treated by BEZ235 with or without chemotherapeutic drugs. A Cell Counting Kit-8 (CCK8, Dojindo Molecular Technologies, Dojindo, Japan) was used to measure AML cell proliferation according to the manufacturer's directions. The absorbance was read at 450 nm in a 630 Microplate Reader (Bio-Rad). Cell growth inhibition rate =1–(experimental group OD value/control group OD value) ×100%.
Cell apoptosis assay
The cells (2×105) were stained with 5 μL of Annexin V-FITC and PI (BioVision, Palo Alto, CA, USA), according to the manufacturer's protocol. The FCM data were analyzed using the QuantiCALC system for flow cytometry (Becton Dickinson, San Jose, CA, USA).
Cell migration assay
Transwell assays were performed using 24-well transwell cell culture inserts with 8 μm pores (Corning). The matrigel was added to the inserts 4 h before the cells were plated into the inserts. Cells treated with different concentrations of BEZ235 were plated at 1×106/mL in the upper chamber. After incubating for 24 h, non-migrating cells were removed from the top well, while the cells in the bottom well were collected and counted by trypan blue exclusion assays in triplicate.
Detection of differentially expressed miRNAs by miRNA microarray
After treatment with or without BEZ235 at IC50 values for 24 h, K562/ADR cells were harvested and subsequently analyzed using a miRNA microarray (Kangchen Bio-tech Company, Shanghai, China). miRNAs were extracted using a miRNeasy FFPE Kit (Qiagen) following the manufacturer's instructions and were analyzed with an ND-1000 spectrophotometer (Nanodrop Technologies Inc, Wilmington, DE, USA). The samples were labeled with a miRCURYTM Hy3TM/Hy5TM Power Labeling Kit (Exiqon) and hybridized to a miRCURYTM LNA Array (Exiqon, v11.0). The results were scanned with an Axon GenePix 4000B microarray scanner and analyzed with GenePix Pro v6.0.
Bioinformatics prediction and luciferase reporter assays
To predict the direct targets of miR-1-3p, we used the common software TargetScan (www.targetscan.org). Both wild type (WT) and the mutants (MT) of the 3′-UTRs of three targets, endothelin 1 (EDN1), Bcl-2-associated athanogene4 (BAG4) and ATP binding cassette subfamily B member 1 (ABCB1), were cloned and inserted into the psi-Check2 plasmid (Promega, USA). Then, all constructs were transfected into HEK-293T cells with 50 nmol/L of miR-1-3p mimics using Lipofectamine 2000 for 5 h. After changing the fresh medium for 24 h, the luciferase activity was calculated using an automatic microplate reader.
QRT-PCR assay
After extracting total RNA using TRIzol reagent, we detected miR-1-3p, its targets, and the internal references U6 and GAPDH using a PrimeScript miRNA RT-PCR Kit (TaKaRa, Dalian, China) or a standard SYBR-Green RT-PCR Kit (TaKaRa) according to the manufacturer's specifications. The specific primer pairs were as follows (Table 1). The relative expression was determined using the 2−ΔΔCt method.
Western blot analysis
Cells were washed in PBS and were solubilized in cold radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific) to extract total proteins. The lysates were boiled for 5 min, separated by 10% SDS-PAGE (Pierce, Rockford, IL, USA) and transferred to polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific). After nonspecific binding sites were blocked for 1 h using 5% skim milk, the membranes were incubated with mouse anti-human EDN1, BAG4, ABCB1 and GAPDH McAb (1:100, Abcam) at 4 °C overnight. Then membranes were washed three times with Tris-buffered saline Tween-20 (TBST) and incubated further for 1 h with the rabbit anti-mouse secondary antibody. The specific binding was visualized by enhanced chemiluminescence reagents. The protein bands were quantified using ImageJ software.
Statistical analysis
All computations were carried out using SPSS version 18.0 for Windows (SPSS, Inc, Chicago, IL, USA). Data are expressed as the mean±SD. One-way ANOVA was used to analyze the differences. Differences were considered statistically significant when P was less than 0.05.
Results
BEZ235 suppressed proliferation and migration and induced apoptosis and chemosensitization in AML cells
The cytotoxic effects of BEZ235 as single agents were assessed in multidrug-resistant AML cell lines. The BEZ235 concentration range used in this experiment was 5-100 nmol/L for 24 h. BEZ235 reduced cell viability in a dose-dependent manner, as shown by CCK8 assays (Figure 1A). The IC50 values of BEZ235 for HL-60/VCR and K562/ADR cells were 66.69 nmol/L and 71.44 nmol/L, respectively. After treatment with different concentrations of BEZ235 for 24 h, the migration of both cell lines significantly decreased (Figure 1B). BEZ235 also induced apoptosis in the HL-60/VCR and K562/ADR cells in a dose-dependent manner (Figure 1C). Multidrug resistance directly affects the outcome of AML, and restoration of drug sensitivity can be attempted by adding drug resistance-modifying agents to standard chemotherapeutic regimens. Here, we used one-tenth IC50 of BEZ235 to sensitize HL-60/VCR and K562/ADR cells to multiple chemotherapeutic agents. As shown in Figure 1D, BEZ235 significantly sensitized cells to chemotherapy by decreasing the IC50 values of VCR and ADR in HL-60/VCR and K562/ADR cells. For HL-60/VCR cells, the IC50 values were from 8.72 to 1.91 μmol/L and from 1.24 to 0.24 μmol/L at 24 h, respectively. For K562/ADR cells, the IC50 values were from 7.52 to 2.24 μmol/L and from 2.27 to 0.41 μmol/L at 24 h, respectively.
BEZ235 regulates cell proliferation, migration, apoptosis, and chemosensitivity in AML cells. (A) After exposure to various concentrations (from 25 to 100 nmol/L) of BEZ235 for 24 h, the viability of HL-60/VCR and K562/ADR cells was determined using CCK8 assays, and the viability decreased in a dose-dependent manner (**P<0.01 compared with the untreated control group). (B) HL-60/VCR and K562/ADR cells treated as above. We collected the cells in the bottom Boyden chamber and counted them using trypan blue exclusion assays. The migration cell count also decreased in a dose-dependent manner (**P<0.01 compared with the untreated control group). (C) Under identical culture conditions, the apoptosis of HL-60/VCR and K562/ADR cells was detected by the FCM method. Opposite results were observed, as both increased in a dose-dependent manner. (D) BEZ235 elevated the sensitivity of HL-60/VCR and K562/ADR cells to VCR and ADR.
BEZ235 up-regulated miR-1-3p expression
To explore the potential mechanisms underlying the effects of BEZ235 on AML multidrug-resistant cells, we identified the miRNAs modulated by BEZ235 at the IC50 in HL-60/VCR cells at 24 h using a miRNA microarray (Figure 2A). We identified 5 up-regulated miRs in HL60/VCR cells and confirmed the changes using QRT-PCR (Figure 2B). miR-1-3p was increased by a factor of 4.33, which represented the most significant and stable up-regulation. Moreover, we detected changes in miR-1-3p in both HL-60/VCR and K562/ADR cells with different concentrations of BZE235 (Figure 2C).
BEZ235 up-regulates miR-1-3p expression. (A) Unsupervised hierarchical clustering of miRNA expression profiles in HL-60/VCR cells treated with IC50 of BEZ235 for 24 h. (B) According to the miR array results, we detected 7 types of up-regulated miRs. (C) HL-60/VCR and K562/ADR cells treated with the indicated concentrations of BEZ235 for 24 h. Total RNA was extracted, and the miR-1-3p expression level was measured by qRT-PCR. **P<0.01.
miR-1-3p negatively regulated EDN1, BAG4 and ABCB1 and had the same effect as BEZ235 on AML cells
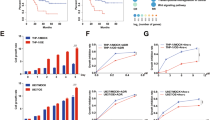
Numerous studies have identified miR-1-3p as a tumor suppressor16,17,18. In this study, using TargetScan analysis, we predicted that EDN1, BAG4 and ABCB1 had potential miR-1-3p target sites in their 3′-UTRs. The seed sequences for miR-1-3p in the 3′-UTRs of these targets are shown in Figure 3A. To confirm the direct interaction of miR-1-3p and its targets, we cloned the WT or MUT target response elements into the psiR-CHECK2 plasmid downstream of the luciferase reporter. After transfecting HEK293T cells with miR-1-3p-MIMIC and the 3′-UTR vectors, we found that the luciferase reporter activity of the WT but not the MUT Check2-EDN1, BAG4 and ABCB1 were inversely correlated with the miR-1-3p expression level (Figure 3B). In the WT group, the activity was approximately 21% to 33% that of the control group (P<0.01). To determine the roles of EDN1, BAG4 and ABCB1 downstream of miR-1-3p, we measured their levels in HL-60/VCR and K562/ADR cells using qRT-PCR (Figure 3C) and Western blot (Figure 3D) analyses. As shown in Figure 3C and 3D, after treatment with miR-1-3p-MIMIC and miR-1-3p-AMO for 24 h, the levels of EDN1, BAG4 and ABCB1 were directly related to the level of miR-1-3p. Moreover, up-regulation of miR-1-3p was consistent with the effect of BEZ235. Compared with the NC control group, the miR-1-3p-MIMIC group had decreased cell proliferation and migration (Figure 3E and 3F). Meanwhile, it also induced apoptosis in HL-60/VCR and K562/ADR cells (Figure 3G). Chemosensitivity was assessed, and the IC50 values of miR-1-MIMIC combined with VCR or ADR in HL-60/VCR and K562/ADR cells were 2.51, 0.368, 2.83 and 0.498, respectively (Figure 3H).
miR-1-3p negatively regulates BAG4, EDN1 and ABCB1. (A) Seed sequence of miR-1-3p combined with the 3′-UTRs of BAG4, EDN1 and ABCB1. (B) The reporter construct containing the predicted miR-1-3p binding site in the 3′-UTRs of the targets. Overexpression of miR-1-3p-MIMIC significantly attenuated luciferase activity of the WT-BAG4, EDN1 and ABCB1 3′-UTRs in 293T cells rather than the MUT-BAG4, EDN1 and ABCB1 3′-UTRs. miR-1-3p-MIMIC and miR-1-3p-AMO were transfected into the HL-60/VCR and K562/ADR cells for 24 h, and the mRNA (C) and protein (D) levels of BAG4, EDN1 and ABCB1 were measured by qRT-PCR and Western blot analyses, respectively. **P<0.01.
miR-1-3p negatively regulates BAG4, EDN1 and ABCB1. To evaluate the effect of miR-1-3p on AML cells, we transfected the miR-1-3p-MIMIC or NC into HL-60/VCR and K562/ADR cells for 24 h. (E) The viability of the miR-1-3p-MIMIC group in both HL-60/VCR and K562/ADR cells was significantly lower than that in the NC group (**P<0.01). (F) The migration assays showed fewer cells in the bottom chamber in the miR-1-3p-MIMIC group than those in the NC group (**P<0.01). (G) Apoptosis in the miR-1-3p-MIMIC group in both HL-60/VCR and K562/ADR cells was significantly higher than that in the NC group. (H) Chemosensitivity in miR-1-3p-MIMIC group in both HL-60/VCR and K562/ADR cells was increased.
Inhibition of miR-1-3p in AML multidrug-resistant cells reduces the effect of BEZ235
After transfection with miR-1-3p-AMO or NC for 12 h, we treated the HL-60/VCR and K562/ADR cells with IC50 values of BEZ235. As shown in Figure 4A, up-regulation of miR-1-3p significantly increased the viability of HL-60/VCR and K562/ADR cells treated with BEZ235 combined with NC. Compared with the NC group, the inhibition of the effects of BEZ235 on expression of EDN1, BAG4 and ABCB1 mRNAs and proteins was attenuated by up-regulating miR-1-3p (Figure 4C and 4D). We also detected apoptosis in HL-60/VCR and K562/ADR cells. In the miR-1-3p-AMO group, the cellular apoptosis was substantially lower than that in the NC group (Figure 4E). Similar trends were observed for chemosensitivity (Figure 4F). After transfection with NC or miR-1-AMO, the IC50 values of VCR or ADR combined with BEZ235 in HL-60/VCR and K562/ADR cells were 1.8 vs 5.91, 0.225 vs 0.794, 2.12 vs 5.31 and 0.331 vs 1.01. These results indicate that miR-1-3p expression plays an important role in the effects of BEZ235 on AML multidrug resistance.
miR-1-3p is involved in the effect of BEZ235 on AML cells. HL-60/VCR and K562/ADR cells were transfected with miR-1-3p-AMO or NC for 24 h and treated with IC50 of BEZ235 for 24 h. (A) The cell viability was measured by CCK8 assays, and the viability of the miR-1-3p-AMO group in both HL-60/VCR and K562/ADR cells was significantly higher than that in the NC group (**P<0.01). (B) Migration was detected using transwell assays, and the results were similar to those of the proliferation assays (**P<0.01). (C) The inhibitory effects of BEZ235 on BAG4, EDN1, and ABCB1 mRNA expression was attenuated by miR-1-3p-AMO. (D) The effects of BEZ235 on BAG4, EDN1, and ABCB1 protein expression was attenuated by miR-1-3p-AMO. (E) Apoptosis assays were performed using Annexin V/PI staining, and the apoptotic ratio in the miR-1-3p-AMO group in both HL-60/VCR and K562/ADR cells was substantially lower than that in the NC group. (F) Chemosensitivity in the miR-1-3p-AMO group in both HL-60/VCR and K562/ADR cells was significantly lower than that in the NC group.
Discussion
The prevalence of PI3K/Akt/mTOR overactivation in tumors indicates an important role for this pathway in cancer pathogenesis and furthermore provides strong support for the therapeutic anticancer application of PI3K/Akt/mTOR inhibitors. BEZ235, an imidazo-quinoline derivative, is a structure-based design compound that strongly inhibits the four PI3K paralogues and directly inhibits the mTOR kinase. BEZ235 also significantly reduced the phosphorylation of the mTOR activated kinase p70S6K. Numerous preclinical studies have shown that NVP-BEZ235 has beneficial pharmaceutical properties as an anticancer reagent. Here, we found that BEZ235 also possesses strong antileukemic activity using CCK8 assays. Both HL-60/VCR and K562/ADR cells showed a dose-dependent reduction in cell proliferation with an IC50 of 66.69 nmol/L and 71.44 nmol/L, respectively. Consistent with its antiproliferative effects, cell apoptosis analysis using Annexin V and PI staining showed a significant increase in the proportion of both apoptotic and dying cells. The migration assays also indicated that the motility of leukemia cell lines was reduced in a dose-dependent manner. As the population ages, the incidence of AML in the elderly increases every year. Older patients and those with comorbidities are often considered ineligible for standard induction therapy, which may result in multidrug resistance and poor outcomes. First-generation mTOR inhibitors, such as rapamycin, have been used to reverse the multidrug resistance in various tumors, including AML. Here, we report for the first time the effects of BEZ235 on chemosensitivity in AML. Our data showed that combination therapy of BEZ235 with different concentrations of VCR and ADR in HL-60/VCR and K562/ADR cells increased the sensitivity of multidrug-resistant leukemia cells to chemotherapeutic drugs. First, we verified the pharmacological effects of the PI3K/mTOR dual inhibitor BEZ235 on AML and showed that it significantly enhanced the anticancer drug activity. However, the underlying mechanism was unknown. MicroRNAs (miRNAs), a new class of small, non-coding RNAs, are critical post-transcriptional regulators of gene expression and have also been shown to play important roles in diverse biological processes, including development, cell differentiation, proliferation, and apoptosis. Accumulating evidence indicates that deregulation of microRNAs is often associated with human malignancies. Microarray platforms enable high-throughput miRNA profiling, and we used these microarrays to identify altered miRNAs after treatment with BEZ235. Our data identified diverse miRs using microarray analysis. Similar to the qRT-PCR results, miR-1-3p showed the most significant increase. miR-1-3p has been considered a tumor suppressors since 2008. Previous studies reported that 5-azacytidine, an epigenetic drug, hypomethylated miR-1-3p and activated several miR-1-3p targets, including FoxP1, MET, and HDAC4, in HCC cell lines and in primary human HCCs, which suppress hepatocarcinoma cell growth19. Nasser et al also found that miR-1-3p was up-regulated in human primary lung cancer tissues and cell lines. Expression of miR-1-3p in nonexpressing A549 and H1299 cells reversed their tumorigenic properties, such as growth, replication potential, motility/migration, clonogenic survival, and tumor formation, in nude mice and sensitized the lung cancer cells to doxorubicin20. Here, we predicted EDN1, BAG4 and ABCB1 as direct targets of miR-1-3p with TargetScan. BAG4/silencer of death domains (SODD) is a member of the BAG family21. BAG proteins are molecular chaperone regulators that affect diverse cellular pathways and comprise two subfamilies; one includes BAG3, BAG4, and BAG5, and the other is represented by BAG1. BAG4 is a widely expressed protein of approximately 60 kilodaltons, and each helix in this bundle is three to four turns shorter than its counterpart in BAG1, which reduces the length of the domain by one-third. BAG-4/SODD is physically associated with Bcl-2, and BAG domains appear to constitute the Bcl-2 binding regions of this molecule. Moreover, BAG-4/SODD, similar to BAG-1, binds tumor necrosis factor receptor type 1 (TNF-R1) monomers through its BAG domain and blocks TNF-alpha-induced apoptosis22,23. BAG4 influences the growth of several types of cancer, such as pancreatic24, ovarian25, and breast cancer26. Additionally, Cisterne27 and Tao28 found that in acute lymphoblastic leukemia, BAG4/SODD plays the important role in various processes, including apoptosis and the chemotherapy sensitivity. We predicted and verified that BAG4 is a direct targets of miR-1-3p and may be a key factor in BEZ235-induced apoptosis. EDN1 is a target of miR-1-3p believed to participate in the BEZ235-inhibited AML cell migration. EDN1 is located on chromosome 6p23-p24. Hyter reported that p53 directly regulates Edn1 expression in epidermal keratinocytes and controls UV-induced melanocyte homeostasis29. Alexander showed that Wnt signaling interacts with BMP and EDN1 to regulate dorsal-ventral patterning and growth of the craniofacial skeleton30. Lu's research indicated that overexpression of endothelin 1 triggers hepatocarcinogenesis and promotes cell proliferation and migration through the AKT pathway31. Here, we found that BEZ235 inhibited AML cell migration via decreases in EDN1 by blocking both PI3K and mTOR through miR-1-3p. For AML patients, chemotherapy is the major treatment modality. However, many patients develop resistance to chemotherapy drugs later in their lives. The appearance of cell populations resistant to multidrug-based chemotherapy constitutes the major obstacle in the treatment of these patients. Recent studies showed that PI3K/Akt/mTOR could regulate the sensitivity of cancer cells to radiotherapy and chemotherapy32. Dinner also reported that the PI3K/Akt/mTOR axis is frequently activated in acute myelogenous leukemia (AML) patient blasts and strongly contributes to drug resistance33. The ABCB1 gene encodes the multidrug transporter P-glycoprotein (P-gp), which is expressed on normal CD34-positive bone marrow cells, indicating hematopoietic stem cells, and other the daughter cells, including some lymphoid cells. Multidrug resistance mediated by P-gp appears to be a major impediment to successful treatment of AML34. The role of P-gp in normal and malignant hematopoiesis and clinical attempts to circumvent multidrug resistance in hematopoietic malignancies have been widely researched35,36. The present study evaluated the in vitro cytotoxic activity of BEZ235 in combination with VCR and ADR in multidrug-resistant AML cell lines. We found that BEZ235 significantly sensitized cells to chemotherapy by decreasing the IC50 values of all drugs in HL-60/VCR and K562/ADR cells. However, BEZ235 increased chemosensitivity via unknown mechanisms. In the present study, we clarified that ABCB1, a major factor of MDR, was a direct target of miR-1-3p.
In conclusion, our study clearly demonstrated that BEZ235 inhibited the proliferation and migration and promoted the chemosensitivity in AML cell lines, through miR-1-3p elevation, thus negatively regulating the expression of BAG4, END1 and ABCB1.
Author contribution
Xiu-ju WANG designed the research; Lan DENG performed the research; Ling JIANG performed the data analysis; Xiang-hua LIN prepared the figures; Kuo-Fu TSENG provided new reagents; Yuan LIU analyzed the data; Xing ZHANG polishes the paper; Rui-hong DONG wrote the pape; Zhi-gang LU polished and modified the paper.
References
Kang ZJ, Liu YF, Xu LZ, Long ZJ, Huang D, Yang Y, et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer 2016; 35: 48.
Khaled SA, Malki M, Marcucci G . Acute myeloid leukemia: biologic, prognostic, and therapeutic insights. Oncology (Williston Park) 2016; 30: 318–29.
Greaves M . Leukaemia 'firsts' in cancer research and treatment. Nat Rev Cancer 2016; 16: 163–72.
Feldman EJ . Novel therapeutics for therapy-related acute myeloid leukemia: 2014. Clin Lymphoma Myeloma Leuk 2015; 15: S91–3.
Brotelle T, Bay JO . PI3K-AKT-mTOR pathway: Description, therapeutic development, resistance, predictive/prognostic biomarkers and therapeutic applications for cancer. Bull Cancer 2016; 103: 18–29.
Morgensztern D, McLeod HL . PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 2005; 16: 797–803.
Hay N . The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005; 8: 179–83.
Tong X, Pelling JC . Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer Agents Med Chem 2013; 13: 971–8.
Dos Santos C, Récher C, Demur C, Payrastre B . The PI3K/Akt/mTOR pathway: a new therapeutic target in the treatment of acute myeloid leukemia. Bull Cancer 2006; 93: 445–7.
Park S, Chapuis N, Saint Marcoux F, Recher C, Prebet T, Chevallier P, et al. A phase Ib GOELAMS study of the mTOR inhibitor RAD001 in association with chemotherapy for AML patients in first relapse. Leukemia 2013; 27: 1479–86.
Shull AY, Noonepalle SK, Awan FT, Liu J, Pei L, Bollag RJ, et al. RPPA-based protein profiling reveals eIF4G overexpression and 4E-BP1 serine 65 phosphorylation as molecular events that correspond with a pro-survival phenotype in chronic lymphocytic leukemia. Oncotarget 2015; 6: 14632–45.
Wong J, Welschinger R, Hewson J, Bradstock KF, Bendall LJ . Efficacy of dual PI3K and mTOR inhibitors in vitro and in vivo in acute lymphoblastic leukemia. Oncotarget 2014; 5: 10460–72.
Park H, Kim Y, Sul JW, Jeong IG, Yi HJ, Ahn JB, et al. Synergistic anticancer efficacy of MEK inhibition and dual PI3K/mTOR inhibition in castration-resistant prostate cancer. Prostate 2015; 75: 1747–59.
Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis 2014; 5: e1437.
Hall CP, Reynolds CP, Kang MH . Modulation of glucocorticoid resistance in pediatric T-cell acute lymphoblastic leukemia by increasing BIM expression with the PI3K/mTOR inhibitor BEZ235. Clin Cancer Res 2016; 22: 621–32.
Stope MB, Hettenbach D, Kaul A, Paditz M, Diesing K, Burchardt M, et al. The tumor suppressor microRNA-1 exhibits restricted inhibition of proliferation of ovarian cancer cells. Anticancer Res 2016; 36: 3329–34.
Jiang S, Zhao C, Yang X, Li X, Pan Q, Huang H, et al. miR-1 suppresses the growth of esophageal squamous cell carcinoma in vivo and in vitro through the downregulation of MET, cyclin D1 and CDK4 expression. Int J Mol Med 2016; 38: 113–22.
Chen X, Shi J, Zhong J, Huang Z, Luo X, Huang Y, et al. miR-1, regulated by LMP1, suppresses tumour growth and metastasis by targeting K-ras in nasopharyngeal carcinoma. Int J Exp Pathol 2015; 96: 427–32.
Datta J, Kutay H, Nasser MW, Nuovo GJ, Wang B, Majumder S, et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res 2008; 68: 5049–58.
Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, et al. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem 2008; 283: 33394–405.
Briknarová K, Takayama S, Homma S, Baker K, Cabezas E, Hoyt DW, et al. BAG4/SODD protein contains a short BAG domain. J Biol Chem 2002; 277: 31172–8.
Takada H, Chen NJ, Mirtsos C, Suzuki S, Suzuki N, Wakeham A, et al. Role of SODD in regulation of tumor necrosis factor responses. Mol Cell Biol 2003; 23: 4026–33.
Cisterne A, Baraz R, Khan NI, Welschinger R, Basnett J, Fung C, et al. Silencer of death domains controls cell death through tumour necrosis factor-receptor 1 and caspase-10 in acute lymphoblastic leukemia. PLoS One 2014; 9: e103383.
Ozawa F, Friess H, Zimmermann A, Kleeff J, Büchler MW . Enhanced expression of Silencer of death domains (SODD/BAG-4) in pancreatic cancer. Biochem Biophys Res Commun 2000; 271: 409–13.
Annunziata CM, Kleinberg L, Davidson B, Berner A, Gius D, Tchabo N, et al. BAG-4/SODD and associated antiapoptotic proteins are linked to aggressiveness of epithelial ovarian cancer. Clin Cancer Res 2007; 13: 6585–92.
Perry NA, Shriver M, Mameza MG, Grabias B, Balzer E, Kontrogianni-Konstantopoulos A . Loss of giant obscurins promotes breast epithelial cell survival through apoptotic resistance. FASEB J 2012; 26: 2764–75.
Cisterne A, Baraz R, Khan NI, Welschinger R, Basnett J, Fung C, et al. Silencer of death domains controls cell death through tumour necrosis factor-receptor 1 and caspase-10 in acute lymphoblastic leukemia. PLoS One 2014; 9: e103383.
Tao HF, Liu YS, Fang JL, Su YZ, Chen FH, Zhou LY, et al. Significance of SODD expression in childhood acute lymphoblastic leukemia and its influence on chemotherapy. Genet Mol Res 2014; 13: 2020–31.
Hyter S, Coleman DJ, Ganguli-Indra G, Merrill GF, Ma S, Yanagisawa M, et al. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res 2013; 26: 247–58.
Alexander C, Piloto S, Le Pabic P, Schilling TF . Wnt signaling interacts with BMP and EDN1 to regulate dorsal-ventral patterning and growth of the craniofacial skeleton. PLoS Genet 2014; 10: e1004479.
Lu JW, Liao CY, Yang WY, Lin YM, Jin SL, Wang HD, et al. Overexpression of endothelin 1 triggers hepatocarcinogenesis in zebrafish and promotes cell proliferation and migration through the AKT pathway. PLoS One 2014; 9: e85318.
Wang Z, Huang Y, Zhang J . Molecularly targeting the PI3K-Akt-mTOR pathway can sensitize cancer cells to radiotherapy and chemotherapy. Cell Mol Biol Lett 2014; 19: 233–42.
Dinner S, Platanias LC . Targeting the mTOR pathway in leukemia. J Cell Biochem 2016; 117: 1745–52.
Bunting KD, Zhou S, Lu T, Sorrentino BP . Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood 2000; 96: 902–9.
Shtil AA . Emergence of multidrug resistance in leukemia cells during chemotherapy: mechanisms and prevention. J Hematother Stem Cell Res 2002; 11: 231–41.
Calado RT, Falcão RP, Garcia AB, Gabellini SM, Zago MA, Franco RF . Influence of functional MDR1 gene polymorphisms on P-glycoprotein activity in CD34+ hematopoietic stem cells. Haematologica 2002; 87: 564–8.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Guangdong Province, China (No S2012010008748 and S2013010014715).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Deng, L., Jiang, L., Lin, Xh. et al. The PI3K/mTOR dual inhibitor BEZ235 suppresses proliferation and migration and reverses multidrug resistance in acute myeloid leukemia. Acta Pharmacol Sin 38, 382–391 (2017). https://doi.org/10.1038/aps.2016.121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.121
Keywords
This article is cited by
-
The important role of miR-1-3p in cancers
Journal of Translational Medicine (2023)
-
Anti-leukemia effects of omipalisib in acute myeloid leukemia: inhibition of PI3K/AKT/mTOR signaling and suppression of mitochondrial biogenesis
Cancer Gene Therapy (2023)
-
Sulfur-oxidizing bacteria (SOB) and sulfate-reducing bacteria (SRB) in oil reservoir and biological control of SRB: a review
Archives of Microbiology (2023)
-
TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling
Cell Death & Disease (2021)
-
PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers
Cell Death & Disease (2020)