Abstract
Aim:
Rosiglitazone is one of the specific PPARγ agonists showing potential therapeutic effects in asthma. Though PPARγ activation was considered protective in inhibiting airway inflammation and remodeling in asthma, the specific mechanisms are still unclear. This study was aimed to investigate whether heme oxygenase-1 (HO-1) related pathways were involved in rosiglitazone-activated PPARγ signaling in asthma treatment.
Methods:
Asthma was induced in mice by multiple exposures to ovalbumin (OVA) in 8 weeks. Prior to every OVA challenge, the mice received rosiglitazone (5 mg/kg, po). After the mice were sacrificed, the bronchoalveolar lavage fluid (BALF), blood samples and lungs were collected for analyses. The activities of HO-1, MMP-2 and MMP-9 in airway tissue were assessed, and the expression of PPARγ, HO-1 and p21 proteins was also examined.
Results:
Rosiglitazone administration significantly attenuated airway inflammation and remodeling in mice with OVA-induced asthma, which were evidenced by decreased counts of total cells, eosinophils and neutrophils, and decreased levels of IL-5 and IL-13 in BALF, and by decreased airway smooth muscle layer thickness and reduced airway collagen deposition. Furthermore, rosiglitazone administration significantly increased PPARγ, HO-1 and p21 expression and HO-1 activity, decreased MMP-2 and MMP-9 activities in airway tissue. All the therapeutic effects of rosiglitazone were significantly impaired by co-administration of the HO-1 inhibitor ZnPP.
Conclusion:
Rosiglitazone effectively attenuates airway inflammation and remodeling in OVA- induced asthma of mice by activating PPARγ/HO-1 signaling pathway.
Similar content being viewed by others
Introduction
Resulted from activation of inflammatory cells including eosinophils, neutrophils, macrophages, mast cells, and T lymphocytes, asthma is now generally accepted as a chronic airway inflammatory disorder1. Under pathological condition of asthma, inflammatory mediators such as cytokines and chemokines, would be released from these cells to induce sustained chronic airway inflammation which eventually leads to bronchoconstriction and airway remodeling2. Stimulated by chronic inflammation, elevated smooth muscle cell proliferation and extracellular matrix (ECM) deposition3,4 would lead to alteration of molecular constitute composition and reorganization of airway wall structure which are the characteristics of airway remodeling5. Furthermore, previous studies suggested airway remodeling is one of the risk factors for corticosteroid-refractory (CSR) asthma6,7. Thus, drugs and reagents which could suppress airway inflammation and remodeling are of therapeutic significance.
Peroxisome proliferator-activated receptors (PPARs) belong to the ligand-activated nuclear receptor superfamily. Three isoforms of PPAR have been cloned, namely the PPAR-alpha (PPARα), PPAR gamma (PPARγ), and PPAR-delta (PPAR&δ)8. Distributed in different organs and tissues, these receptors play important roles in critical biological processes including glucose and lipid homeostasis regulation, cell proliferation, cell differentiation and inflammation9. Strategy of activating PPARγ has shown benefits in protecting cell from inflammation-induced injuries. Rosiglitazone was identified as one of the specific agonists of PPARγ which exerted potential therapeutic effects on acute lung injury and pulmonary arterial hypertension according to previous studies of our group10,11. A study suggested that rosiglitazone prevented perinatal nicotine exposure- induced asthma in rat offspring12. It was reasonable for us to presume that rosiglitazone could alleviate airway remodeling by activating PPARγ in asthma.
According to previous literatures, heme oxygenase (HO)-1 was suggested as one of the down-stream effectors of PPARγ11,13. Our previous work showed that HO-1 could up-regulate expression of p21 protein10 which was considered as an inhibitor of cell proliferation. Notably, matrix metalloproteinase (MMP)2 and MMP-9 were considered regulated by HO-1, which are the key molecules adjusting ECM deposition and degradation in airway remodeling. Thus, PPARγ/HO-1/MMPs and PPARγ/HO-1/p21 are the possible pathways in regulating airway remodeling in asthma. In this study, in vivo model of asthma induced by ovalbumin (OVA) administration was used to evaluate the therapeutic effects of rosiglitazone. Furthermore, the possible involvement of PPARγ/HO-1/MMPs and PPARγ/HO-1/p21 pathways in rosiglitazone's anti-asthma mechanism was also investigated.
Materials and methods
Animals and in vivo asthma model
Thirty-two female Balb/c mice (6 week-old, SPF class, provided by Experimental Animal Center of Xi'an Jiaotong University) were maintained in separated cages under controlled condition (temperature 25±1.5 °C, humidity 65%±4%, 12-h light- dark artificial cycle) for 7 d prior to subsequent experiments. Mice could access freely to fresh water and standard diet continuously. The animal experimental procedures followed protocols approved by Medical Animal Research Ethics Committee at Xi'an Jiaotong University.
Thirty-two mice were divided into 4 groups evenly and randomly: control group (Con), OVA-induced asthma group (OVA), rosiglitazone treated OVA-induced asthma group (OVA+ROSI) and rosiglitazone and zinc protoporphyrin-IX (ZnPP) treated OVA-induced asthma group (OVA+ROSI+ZnPP). For in vivo asthma modeling, mice were initially sensitized by intraperitoneal injection of 0.1 mL PBS supplemented with 25 μg OVA (Sigma-Aldrich) and 500 μg alum (Sigma-Aldrich) at d 1, 7, and 14. Then in the following 8 weeks, OVA (15 μg) was administered intranasally 3 days per week. For rosiglitazone treatment, rosiglitazone (GSK, 5 mg/kg) was administered orally 6 h prior to every OVA intranasal challenge14. For ZnPP treatment, ZnPP (Sigma-Aldrich, 10 mg/kg, dissolved in 5 mmol/L NaOH, pH=7.4) was administered intraperitoneally accompanied with every rosiglitazone treatment.
Serum and bronchoalveolar lavage fluid (BALF) collection
After mice were sacrificed by overdose anaesthetization of pentobarbital sodium (100 mg/kg), blood sample was collected. Serum was collected after blood was centrifuged at 1200×g for 15 min at room temperature which was then stored at -80 °C. Cold sterile PBS was used to perform bronchoalveolar lavage (BAL) by intratracheal injection. Then the PBS was collected as BALF and the average fluid recovery rate was greater than 90%. BALF was centrifuged at 800×g for 10 min at 4 °C. The resulted pellet cells were subjected to differential cell count after Wright Giemsa staining. Total cell, eosinophils and neutrophils were counted under a light microscope. The result supernatant was stored at -80 °C for subsequent enzyme- linked immunosorbent assay (ELISA).
Histological assessment
Immediately after the mice were sacrificed, the right lung was lavaged by fixation solution (0.8% formalin and 4% acetic acid) intratracheally. After harvested, lung was further fixed by 10% (v/v) neutral buffered formalin for 48 h and then embedded with paraffin. The lung was then sliced into sections (4 μm) which were then processed by hematoxylin & eosin (H&E) staining and Masson staining. Spot Colled Color Digital camera (Q Imaging) was used to observe the sections and capture the photomicrograph images. The airway smooth muscle layer thickness and collagen intensities were measured by Un-ScanIt software.
ELISA assay
Mice IL-5 ELISA kit (R&D) and mice IL-13 ELISA kit (R&D) were used to detect IL-5 and IL-13 concentrations in BALF supernatant. In addition, serum OVA-sIgE level was detected by using mice OVA-sIgE ELISA kit (R&D). Experimental protocols were in accordance with manufacturer's instructions.
Western blotting
Harvested left lungs were minced and then homogenized by RIPA lysis buffer (Sigma-Aldrich) supplemented with phosphatase inhibitor and protease inhibitor cocktail on ice. After lysates were centrifuged at 14000×g for 20 min at 4 °C, the resulted supernatant was collected as total protein. A BCA Protein Assay kit (Thermo) was used to determine the concentration of protein sample. Protein (50 μg) was loaded and then separated by vertical electrophoresis on SDS-PAGE gel. The separated protein was transferred electronically to Trans-Blot nitrocellulose membrane (Bio-Rad) which were then incubated with antibodies against PPARγ (Abcam), HO-1 (Cell Signaling Tech), p21 (BD Pharmingen), and β-Actin (Abcam). After washing, the membranes were incubated with corresponding horseradish peroxidase-conjungated secondary antibodies (Sigma-Aldrich). Finally, the membranes were developed by SuperSignal West Pico Chemiluminescent Substrate (Pierce Protein Biology) and exposed to X-ray films. The intensities of the immunoblots were quantified by Scion Image software.
HO-1 enzymatic activity assay
The tissue homogenates was centrifuged at 10000×g for 15 min at 4 °C. The resulted supernatant was treated as testing sample. The enzymatic activity of HO-1 was evaluated by using HO-1 activity assay kit (GenMed) according to the manufacturer's instructions. This spectrophotometric method was based on the conversion of heme into carbon monoxide, biliverdin and ferrous iron.
MMP activity determination by zymography
The extracted protein from lung tissue was mixed with Tris- glycine SDS sample buffer without reducing agents and separated in gelatin zymography gel by vertical electrophoresis. Then the gel was incubated in 2.5% Triton X-100 for 30 min, followed by incubation in Tris-HCl (10 mmol/L, pH=7.4) supplemented with 0.2 mol/L NaCl, 5 mmol/L CaCl2 and 0.02% Brij 35. 0.5% Coomassie blue R250 was used to stain the gel which was destained in destaining buffer (containing 25% MeOH and 10% acetic acid). The resulted bands were visualized and analyzed by Gel DocTM XR (Bio-Rad).
Statistics
Values in this study are presented in a (mean±SD) manner. Data was analyzed by SPSS (SPSS, ver 16.0) statistic software. Differences were analyzed using one-way ANOVA followed by Tukey's post-hoc tests. P<0.05 was considered statistically significant.
Results
Rosiglitazone treatment attenuated airway inflammation in OVA- induced asthma model
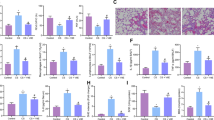
Both of eosinophils cell count and inflammatory cytokine detection were used to investigate the effects of rosiglitazone in asthma mice. The cell count of total cell, eosinophils and neutrophils elevated dramatically in OVA compared with Con in BALF. Eosinophils increase is generally considered as the hall mark of asthma onset and neutrophils is considered the indicator of inflammation (Figure 1). Rosiglitazone administration in ROSI significantly reduced inflammatory cell accumulation in BALF. Moreover, also demonstrated in Figure 1, levels of inflammatory cytokines including IL-5, IL-13, and OVA-sIgE increased significantly in OVA-induced asthma mice which were reduced by ROSI significantly. Notably, ZnPP administration dramatically impaired rosiglitazone's inhibitory effects on eosinophils accumulation and inflammatory cytokine elevation.
Columns indicate total cell count (A), eosinophil cell count (B) and neutrophil cell count (C) in every 104 cells/mL. (D) Columns indicate the serum OVA-sIgE concentration in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP respectively. (E and F) columns indicate the IL-5 and IL-13 concentrations detected in BALF in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP respectively. Values are presented in (mean±SD) manner in column graphs. bDifferences are significant when compared with Con. eDifferences are significant when compared with OVA. hDifferences are significant when compared with OVA+ROSI.
Rosiglitazone treatment alleviated airway remodeling in OVA- induced asthma model
Collagen deposition and airway smooth muscle layer thickness were investigated by H&E and Masson's staining, respectively to indicate the degree of airway remodeling. Figure 2 demonstrated the captured images of H&E and Masson's staining of airway tissue. Collagen deposition and airway smooth muscle layer thickness were significantly increased in OVA-treated mice but then alleviated remarkably by rosiglitazone treatment. However, in OVA+ROSI+ZnPP, after administration of ZnPP, the HO-1 inhibitor, collagen deposition and airway smooth muscle layer thickness were more increased than in OVA+ROSI group.
The upper panel of this figure demonstrated H&E and Masson's staining of airway tissue slice from Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. On the lower panel, columns on the left part indicate the measured smooth muscle layer thickness by observing H&E stained slices in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively; columns on the right part indicate the measured collagen content by observing Masson stained slices in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. Values are presented in (mean±SD) manner in column graphs. bP<0.05 vs Con. eP<0.05 vs OVA. hP<0.05 vs OVA+ROSI.
The effects of rosiglitazone and ZnPP on PPARγ expression in asthma mice
As shown in Figure 3, the expression level of PPARγ was significantly increased in OVA than Con. In OVA+ROSI, further elevated PPARγ expression was found when compared with OVA. Furthermore, there were no significant changes of PPARγ expression after ZnPP administration in OVA+ROSI+ZnPP in comparison to OVA+ROSI group.
The upper part of this figure demonstrated the immunoblots of PPARγ and β-actin (as internal reference) in airway tissue in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. Columns on the lower part of this figure indicate relative expression levels of PPARγ in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. Values are presented in (mean±SD) manner in column graphs. bP<0.05 vs Con. eP<0.05 vs OVA.
The effects of rosiglitazone and ZnPP on HO-1 enzymatic activity and expression
Figure 4 demonstrated HO-1 enzymatic activity and expression level determined by spectrophotometric method and Western blotting respectively in each group. It was found that the HO-1 enzymatic activity was up-regulated in OVA treated mice. In OVA+ROSI, the enzymatic activity was augmented after rosiglitazone administration. As the HO-1 inhibitor, ZnPP treatment dramatically reduced HO-1 enzymatic activity. Also demonstrated in Figure 4, the expression level of HO-1 increased in OVA-treated mice and further increased in mice received co-administration of OVA and rosiglitazone. However, in OVA+ROSI+ZnPP, ZnPP treatment significantly reduced HO-1 expression.
The upper part of this figure demonstrated the immunoblots of HO-1 and β-actin (as internal reference) in airway tissue in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. On the lower panel, columns on the middle part indicate the relative expression of HO-1 in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively; columns on the bottom part indicate the detected HO-1 enzymatic activity in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. Values are presented in (mean±SD) manner in column graphs. bP<0.05 vs Con. eP<0.05 vs OVA. hP<0.05 vs OVA+ROSI.
The effects of rosiglitazone and ZnPP on expressions of p21 and MMPs
As the down- stream effectors of HO-1 which participate in airway remodeling, expression of p21 and activities of MMP-2 and MMP-9 were also examined in this study. Figure 5A demonstrated the expression level of p21 in each group. We found that p21 expression was down-regulated in mice with asthma. However, after administration of rosiglitazone in OVA+ROSI, the p21 expression was significantly up-regulated. ZnPP treatment dramatically reduced p21 expression in OVA+ROSI+ZnPP than OVA+ROSI. Figure 5B demonstrated activities of MMP-2 and MMP-9 detected by zymography. The up-regulated MMP-2 and MMP-9 activities in OVA were impaired by rosiglitazone treatment in OVA+ROSI. Nevertheless, the MMP-2 and MMP-9 activities were elevated after ZnPP administration in OVA+ROSI+ZnPP.
(A) On the upper panel, the immunoblots of p21 and β-actin (as internal reference) in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP were demonstrated. Columns on the lower panel indicate the relative expression levels of p21 in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP respectively. (B) The upper panel of this figure shows the blots of MMP-9 and MMP-2 in gelatin zymography assay. Columns on the lower part indicate the activities of MMP-2 and MMP-9 in Con, OVA, OVA+ROSI, and OVA+ROSI+ZnPP, respectively. Values are presented in (mean±SD) manner in column graphs. bP<0.05 vs Con. eP<0.05 vs OVA. hP<0.05 vs OVA+ROSI.
Discussion
Asthma is a common chronic airway inflammatory disorder due to immune and inflammatory reactions. Airway inflammation and remodeling are the two characterized pathological features of asthma15,16. Among the inflammatory cells participated in occurrence and development of asthma, eosinophils play a featured role17,18. It was considered that the inflammatory mediators and cytokines released by accumulated eosinophils in airway resulted in mucus hypersecretion, increased vascular permeability, bronchial hyperresponsiveness, smooth muscle contraction19,20. Furthermore, cytokines and growth factors released by inflammatory cells such as neutrophils are also involved in initiation of airway remodeling which is characterized by airway smooth muscle proliferation21. In this study, we investigated the therapeutic effect of rosiglitazone in OVA-induced asthma and possible mechanisms involved. We found that rosiglitazone attenuated both airway inflammation and remodeling. The results in this study suggested the therapeutic effects were correlated with activation of PPARγ/HO-1 pathway.
It is believed that PPARγ is a nuclear factor with broad functions in regulating various critical cellular biological processes. PPARγ was originally known as the mediator in regulating cell differentiation and lipid metabolism in adipocytes22. However, subsequent accumulating evidence indicated PPARγ's more extensive functions in regulating cell cycle, apoptosis, inflammation, proliferation and so on23. In this study, we found that PPARγ expression was elevated in OVA- induced asthmatic animal. This finding was in accordance with results from previous study in which up-regulated PPARγ expression was also found in human asthmatic airway24. Previous studies suggested that PPARγ activation alleviated asthmatic features which was evidenced by decreased expressions of cytokines, reduced bronchoconstriction and impaired eosinophils accumulation25,26. Rosiglitazone, one of thiazolidinediones, which is generally considered an agonist for PPARγ with high affinity, was administered to asthmatic animals in this study. Though thiazolidinediones have been withdrawn from the clinical usage in diabetes because of the possible occurrence of cardiovascular complications, they are still of research value because of their property of activating PPARγ. We found that rosiglitazone administration attenuated asthmatic features including airway eosinophils accumulation, inflammatory cytockine levels, smooth muscle layer thickness and collagen deposition, suggesting that the therapeutic effect against asthma exerted by rosiglitazone was associated with activation of PPARγ and its down-stream pathways.
HO-1 is the inducible isoform of HO which catalyzes the degradation of heme into biliverdin, ferrous ion and carbon monoxide27. The enzymatic activity of HO-1 is often stimulated in responses to multiple stimuli such as UV radiation, lipopolysaccharide (LPS), heat shock and heavy metals28. Increased HO-1 activity is found protective against inflammation, excessive cell proliferation and interstitial fibrosis29. Previous study suggested HO-1 could protect against airway inflammation by down-regulating tumor necrosis factor receptor (TNFR)1 dependent oxidative stress30. In asthma particularly, it was considered that the increase of HO-1 expression and activity were protective31. The anti-asthmatic effects of several reagents and drugs such as Sanguisorba officinalis L and Ulmus davidiana var japonica were also found associated with up-regulating airway HO-132,33. HO-1 has been identified as the down-stream effectors of PPARγ. In our previous studies, we found activation of PPARγ by rosiglitazone significantly inhibited proliferation of pulmonary artery smooth muscle cells which was associated with induction of HO-110,11. In the present study, HO-1 enzymatic activity was found increased in response to OVA administration, which was found further elevated in rosiglitazone treated asthmatic mice. After treated by HO-1 inhibitor ZnPP, however, HO-1 activity was reduced dramatically but ZnPP did not affect the PPARγ expression in return. These results suggested that HO-1 was a key mediator in conducting PPARγ signaling to exert protective and therapeutic effect in OVA-induced asthma. We also investigated the translational expression level of HO-1. The results turned out that HO-1 expression was increased in OVA-induced asthmatic mice and further increased by rosiglitazone treatment. Notably, the ZnPP treatment reduced HO-1 expression. There was an inducing effect of ZnPP on expression of HO-1 via early growth response-1 (Egr-1) protein consensus sequence34. However, several other studies suggested ZnPP treatment reduced HO-1 protein expression under certain stressful conditions which was in accordance with our results35,36. Indeed, there are conflicting results and arguments about the role of ZnPP in regulating HO-1 expression and the related mechanisms are complicated. We presume it is possible that the effect of ZnPP on HO-1 expression differs under different pathological conditions and further studies are needed.
Several studies showed that this anti-proliferation effect of HO-1 was correlated with up-regulation of its down- stream molecule p2137 which was believed with proliferation inhibitory effect38. Results in this study indicated that after PPARγ/HO-1 pathway was activated by rosiglitazone treatment in asthmatic mice, p21 expression was correspondingly increased. As a result, airway smooth muscle layer thickness which indicated the smooth muscle proliferation was reduced remarkably. In order to reinforce our finding, after the HO-1 activity was inhibited by ZnPP, p21 expression was also inhibited which was accompanied by effect of impaired rosiglitazone on reducing airway smooth muscle layer thickness. Airway collagen deposition was considered as another feature of airway remodeling, particularly in the patients with chronic asthma39,40,41. Decreased activities of MMPs were believed associated with collagen deposition because MMPs are capable of degrading extracellular matrix components including collagen42. HO-1 was reported to reduce collagen by decreasing MMP-2/9 activities and increasing tissue inhibitors of metalloproteinase (TIMP) activities43. In this study, we found rosiglitazone-activated PPARγ/HO-1 pathway reduced activities of MMP-2 and MMP-9 and collagen deposition in asthmatic airway, which was reversed by ZnPP administration. These results demonstrated above suggested that p21 and MMP-2/9 were responsible for rosiglitazone-activated PPARγ/HO-1 pathway in reducing airway remodeling in asthmatic mice.
In summary, rosiglitazone showed therapeutic effect by attenuating airway inflammation and remodeling in mice with OVA-induced asthma. Activation of PPARγ/HO-1/p21 and PPARγ/HO-1/MMPs pathways are involved in attenuated airway remodeling by rosiglitzone in mice with OVA-induced asthma.
Author contribution
Jing XU, Xiu-zhen SUN, Yuan LIU, Yun LIU, and Man-xiang LI contributed to the research design; Jing XU performed the experiments and the data analysis with the assistance of Gui-zuo WANG, Yan-ting ZHU, Dong HAN, De-xin ZHANG, Jia-mei LU, Yong-hong ZHANG, Yuan-yuan WU, Xin-ming XIE, Shao-jun LI, Rui KE, Lu LIU, and Wei FENG; Jing XU, Xiu-zhen SUN, and Man-xiang LI contributed to the writing of the manuscript.
References
Erjefalt JS . The airway epithelium as regulator of inflammation patterns in asthma. Clin Respir J 2010; 4 Suppl 1: 9–14.
Hoshino M . Airway inflammation and remodeling in bronchial asthma. Nihon Naika Gakkai Zasshi 2006; 95: 1425–30.
Elias JA, Zhu Z, Chupp G, Homer RJ . Airway remodeling in asthma. J Clin Invest 1999; 104: 1001–6.
Black JL, Roth M, Lee J, Carlin S, Johnson PR . Mechanisms of airway remodeling. Airway smooth muscle. Am J Respir Crit Care Med 2001; 164 (10 Pt 2): S63–6.
Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest 2003; 123 (3 Suppl): 417S–22S.
Holgate ST, Polosa R . The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006; 368: 780–93.
Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, et al. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl 2003; 44: 24s–29s.
Kota BP, Huang TH, Roufogalis BD . An overview on biological mechanisms of PPARs. Pharmacol Res 2005; 51: 85–94.
Lamers C, Schubert-Zsilavecz M, Merk D . Therapeutic modulators of peroxisome proliferator-activated receptors (PPAR): a patent review (2008-present). Expert Opin Ther Pat 2012; 22: 803–41.
Zhang D, Wang G, Han D, Zhang Y, Xu J, Lu J, et al. Activation of PPAR-gamma ameliorates pulmonary arterial hypertension via inducing heme oxygenase-1 and p21(WAF1): an in vivo study in rats. Life Sci 2014; 98: 39–43.
Wang G, Liu L, Zhang Y, Han D, Liu J, Xu J, et al. Activation of PPARgamma attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur J Pharmacol 2014; 726: 27–32.
Liu J, Sakurai R, O'Roark EM, Kenyon NJ, Torday JS, Rehan VK . PPARgamma agonist rosiglitazone prevents perinatal nicotine exposure-induced asthma in rat offspring. Am J Physiol Lung Cell Mol Physiol 2011; 300: L710–7.
Park SY, Bae JU, Hong KW, Kim CD . HO-1 induced by cilostazol protects against TNF-alpha-associated cytotoxicity via a PPAR-gamma-dependent pathway in human endothelial cells. Korean J Physiol Pharmacol 2011; 15: 83–8.
Park SJ, Lee KS, Kim SR, Min KH, Choe YH, Moon H, et al. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol 2009; 183: 3259–67.
Jeffery PK . Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004; 1: 176–83.
Bao ZS, Hong L, Guan Y, Dong XW, Zheng HS, Tan GL, et al. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol 2011; 11: 899–906.
Brusselle GG, Maes T, Bracke KR . Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med 2013; 19: 977–9.
Takatsu K . Allergic inflammation and eosinophils: trials, tribulations and enigma of IL-5, eosinophils and asthma. Arerugi 2005; 54: 48–52.
Smith H . Asthma, inflammation, eosinophils and bronchial hyperresponsiveness. Clin Exp Allergy 1992; 22: 187–97.
Walsh GM, Sexton DW, Blaylock MG . Corticosteroids, eosinophils and bronchial epithelial cells: new insights into the resolution of inflammation in asthma. J Endocrinol 2003; 178: 37–43.
Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 2001; 164: 474–7.
Sakaguchi K, Morita I, Murota S . PPAR (peroxisome proliferator activated receptor), a factor that decides differentiation of adipocytes. Kokubyo Gakkai Zasshi 1997; 64: 458.
Krishnan A, Nair SA, Pillai MR . Biology of PPAR gamma in cancer: a critical review on existing lacunae. Curr Mol Med 2007; 7: 532–40.
Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, Mechighel P, et al. Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J Respir Crit Care Med 2001; 164: 1487–94.
Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, et al. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J Exp Med 2003; 198: 411–21.
Honda K, Marquillies P, Capron M, Dombrowicz D . Peroxisome proliferator-activated receptor gamma is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol 2004; 113: 882–8.
Grochot-Przeczek A, Dulak J, Jozkowicz A . Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 2012; 122: 93–103.
Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, et al. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J 2006; 20: 207–16.
Li M, Li Z, Sun X, Yang L, Fang P, Liu Y, et al. Heme oxygenase-1/p21WAF1 mediates peroxisome proliferator-activated receptor-gamma signaling inhibition of proliferation of rat pulmonary artery smooth muscle cells. FEBS J 2010; 277: 1543–50.
Lee IT, Luo SF, Lee CW, Wang SW, Lin CC, Chang CC, et al. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol 2009; 175: 519–32.
Kitada O, Kodama T, Kuribayashi K, Ihaku D, Fujita M, Matsuyama T, et al. Heme oxygenase-1 (HO-1) protein induction in a mouse model of asthma. Clin Exp Allergy 2001; 31: 1470–7.
Lee NH, Lee MY, Lee JA, Jung DY, Seo CS, Kim JH, et al. Anti-asthmatic effect of Sanguisorba officinalis L and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int J Mol Med 2010; 26: 201–8.
Lee MY, Seo CS, Ha H, Jung D, Lee H, Lee NH, et al. Protective effects of Ulmus davidiana var japonica against OVA-induced murine asthma model via upregulation of heme oxygenase-1. J Ethnopharmacol 2010; 130: 61–9.
Yang G, Nguyen X, Ou J, Rekulapelli P, Stevenson DK, Dennery PA . Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood 2001; 97: 1306–13.
Fei D, Meng X, Zhao M, Kang K, Tan G, Pan S, et al. Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Transl Res 2012; 159: 99–109.
Wang QM, Du JL, Duan ZJ, Guo SB, Sun XY, Liu Z . Inhibiting heme oxygenase-1 attenuates rat liver fibrosis by removing iron accumulation. World J Gastroenterol 2013; 19: 2921–34.
Kumar D, Bhaskaran M, Alagappan L, Tori D, Yadav I, Konkimalla S, et al. Heme oxygenase-1 modulates mesangial cell proliferation by p21 Waf1 upregulation. Ren Fail 2010; 32: 254–8.
Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, Alam J, Nath KA . Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int 2001; 60: 2181–91.
Gueders MM, Foidart JM, Noel A, Cataldo DD . Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol 2006; 533: 133–44.
Oikonomidi S, Kostikas K, Tsilioni I, Tanou K, Gourgoulianis KI, Kiropoulos TS . Matrix metalloproteinases in respiratory diseases: from pathogenesis to potential clinical implications. Curr Med Chem 2009; 16: 1214–28.
Ohbayashi H . Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci 2002; 3: 409–21.
Cauwe B, Van den Steen PE, Opdenakker G . The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol 2007; 42: 113–85.
Shu T, Zeng B, Ren X, Li Y . HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell 2010; 42: 217–22.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No 81170051 and 81330002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Zhu, Yt., Wang, Gz. et al. The PPARγ agonist, rosiglitazone, attenuates airway inflammation and remodeling via heme oxygenase-1 in murine model of asthma. Acta Pharmacol Sin 36, 171–178 (2015). https://doi.org/10.1038/aps.2014.128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2014.128
Keywords
This article is cited by
-
Harnessing peroxisome proliferator-activated receptor γ agonists to induce Heme Oxygenase-1: a promising approach for pulmonary inflammatory disorders
Cell Communication and Signaling (2024)
-
Imbalanced serum levels of resolvin E1 (RvE1) and leukotriene B4 (LTB4) in patients with allergic rhinitis
Molecular Biology Reports (2020)
-
Inhibition of NADPH Oxidase-Derived Reactive Oxygen Species Decreases Expression of Inflammatory Cytokines in A549 Cells
Inflammation (2019)
-
Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model
Acta Pharmacologica Sinica (2018)
-
Activation of Cold-Sensitive Channels TRPM8 and TRPA1 Inhibits the Proliferative Airway Smooth Muscle Cell Phenotype
Lung (2016)