Abstract
Aim:
To investigate the effects of arbidol hydrochloride (ARB), a widely used antiviral agent, on the inflammation induced by influenza virus.
Methods:
MDCK cells were infected with seasonal influenza A/FM/1/47 (H1N1) or pandemic influenza A/Hubei/71/2009 (H1N1). In vitro cytotoxicity and antiviral activity of ARB was determined using MTT assay. BALB/c mice were infected with A/FM/1/47 (H1N1). Four hours later the mice were administered ARB (45, 90, and 180 mg·kg−1·d−1) or the neuraminidase inhibitor oseltamivir (22.5 mg·kg−1·d−1) via oral gavage once a day for 5 d. Body-weight, median survival time, viral titer, and lung index of the mice were measured. The levels of inflammatory cytokines were examined using real-time RT-PCR and ELISA.
Results:
Both H1N1 stains were equally sensitive to ARB as tested in vitro. In the infected mice, ARB (90 and 180 mg·kg−1·d−1) significantly decreased the mortality, alleviated virus-induced lung lesions and viral titers. Furthermore, ARB suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10 in the bronchoalveolar lavage fluids and lung tissues. However, ARB did not significantly affect the levels of IFN-α and IFN-γ, but reduced the level of IFN-β1 in lung tissues at 5 dpi. In peritoneal macrophages challenged with A/FM/1/47 (H1N1) or poly I:C, ARB (20 μmol/L) suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10. Oseltamivir produced comparable alleviation of virus-induced lung lesions with more reduction in the viral titers, but less effective modulation of the inflammatory cytokines.
Conclusion:
ARB efficiently inhibits both H1N1 stains and diminishes both viral replication and acute inflammation through modulating the expression of inflammatory cytokines.
Similar content being viewed by others
Introduction
Outbreaks of influenza cause substantial morbidity and mortality each year. New strains of influenza viruses (IFV) emerge periodically and lead to pandemics that pose a great threat to human health1,2. H1N1 has attracted greater attention because it is responsible for a fraction of annual seasonal influenza infections as well as the pandemic in 2009 and 1918. The recruitment of macrophages and neutrophils into the lung controls the severity of the influenza infection3. However, some IFV strains can initiate an excessive production of inflammatory cytokines, which is associated with lethal disease, cumulating in severe lung injury such as was seen in the 1918 pandemic IFV H1N1 infection4. Presently, two classes of antiviral drugs are used in treating IFV infections: the M2 channel blockers (adamantine and rimantadine) and the neuraminidase inhibitors (oseltamivir and zanamivir)5. However, increasing drug resistance, toxicity and side effects limit the application of these antivirals6,7. It is urgent to develop antivirals with novel pharmacological activities to overcome influenza infection8,9. Antivirals that can reduce both viral replication and lung inflammation would be extremely appreciated.
Arbidol hydrochloride (ARB) is a small monocular compound used as a broad-spectrum inhibitor of influenza A and B virus, parainfluenza virus, respiratory syncytial virus, adenovirus, coxsackievirus and hepatitis C virus infection10,11,12,13,14,15,16,17. It is less likely to induce drug resistance than other compounds, such as adamantine and oseltamivir11,12,16,18. However, its mechanism is complicated: earlier reports indicated that ARB could inhibit the membrane fusion of enveloped viruses in vitro and strengthen the interactions between virus glycoprotein and the membrane of host during the endocytosis17,19,20. However, this scenario cannot explain the antiviral activity of ARB on non-enveloped viruses that directly release genomes into the cytosol without a membrane fusion process14. Moreover, a recent study has found that ARB exhibited only 6% to 8% inhibition of IFV viral fusion from measurements of hemolysis analysis in vitro21. It seems that the antiviral efficacy of ARB does not completely depend on the anti-fusion activity.
It has also been reported that ARB exhibits some immunomodulatory activities, such as the effects of interferon induction and macrophage activation22,23. Interferon levels increases in both cell cultures and animals after ARB treatment23. However, conflict evidence indicates that ARB inhibited IFN-β transcription in a dose-dependent manner during HCV infection24. In clinical observations, ARB not only decreases intoxication and the severity of catarrhal syndrome in acute infections but also exhibits preventive effect on post-influenza complications and reduces the incidence of exacerbations of chronic diseases in post-influenza patients22, which implies an anti-inflammation potential of ARB.
The antiviral effect of ARB on IFV has been described previously12,21, but few studies have paid attention to the anti-inflammatory activity of ARB on IFV infection. Here, we evaluated the antiviral and anti-inflammatory activity of ARB on IFV H1N1 infection in vitro and in vivo. Furthermore, we estimated whether ARB can also inhibit the inflammatory response induced by a virus-like mimic, poly I:C, both in isolated peritoneal macrophages and in an acute inflammatory mice model. This study will help us to explore the utilization of ARB in the prevention of severe pneumonia and virus-associated cytokine dysregulation induced by IFV.
Materials and methods
Chemicals, reagents, and cells
ARB was provided by Shijiazhuang No 4 Pharmaceutical Co Ltd, China. Oseltamivir phosphate (OSE) was purchased from Roche. Chemicals were suspended in 0.5% sterilized methylcellulose for oral gavage. MDCK (Madin-Darby Canine Kidney) cells were obtained from the China Center for Type Culture Collection (CCTCC) and were routinely grown in DMEM with 10% fetal bovine serum (FBS), 0.1% L-glutamine and 100 U/mL penicillin and streptomycin. DMEM containing 2 μg/mL trypsin was used for maintaining the medium after viral infection.
Viruses
Influenza A/Hubei/74/2009 is an influenza strain that was isolated from fever patients in Hubei Province between June and November 2009. Sequence comparison revealed that it shared 99% homology with the known epidemic strain A/California/04/2009 (H1N1)25. Influenza A/FM/1/47 was used as a reference prototype strain in this study. Its mouse-adapted variant has been well studied and widely used in research26. All the IFV strains were kindly provided by Prof Tian-xian LI (Wuhan Institute of Virology, Chinese Academy of Sciences). The virus was propagated in the allantoic cavity of 11-d-old embryonated chicken eggs for 48 h at 35 °C and then for 12 h at 4 °C. The harvested viruses were inoculated in MDCK cells and SPF BALB/c mice for adaptation as described previously26. The culture suspensions and lung homogenates were collected and stored in multiple single-use aliquots at −80 °C for cell infection and animal inoculation. The 50% lethal dose (LD50) of mouse was determined to be 1×104.1 PFU/mL.
In vitro cytotoxicity and antiviral activity assay
The in vitro cytotoxicity and antiviral activity of ARB were determined by quantitative colorimetric MTT assay as described previously14. Briefly, MDCK cells infected with influenza viruses (A/Hubei/74/2009 at 0.07 MOI and A/FM/1/47 at 0.10 MOI) were treated with serially diluted ARB solutions at −6 h (6 h before viral infection, pre-treatment mode), 0 h (at the same time as viral infection, simultaneous treatment mode) or 1 h (1 h after viral infection, post-treatment mode). After incubation for 72 h, the inhibition of virus-induced Cytopathic Effect (CPE) in all groups was measured by the MTT assay. Viral control, normal control and solvent control were included in all assays. Five serial dilutions of ARB (from 38.0 to 2.4 μmol/L) were tested in triplicate. The concentration of drug that reduced the infectious titer by 50% of the median effective dose (EC50) was determined by regression analysis.
Animal experiment design
This study was approved by the Ethics Committee of Wuhan University School of Medicine. All animal researches were performed in the Animal Biosafety Level 3 (ABSL-3) Laboratory of the Animal Research Center at Wuhan University and received humane care in compliance with the Chinese Animal Protection Act and the National Research Council criteria.
Eight-week-old SPF female BALB/c mice obtained from the Animal Center of Wuhan University were randomly assigned to 6 groups. The mice were anesthetized intraperitoneally by ketamine (100 mg/kg) and intranasally inoculated with 50 μL viral suspension containing 10 LD50 of influenza A/FM/1/47 (H1N1) virus (mouse adapted) or PBS in the normal control group. As the 50% lethal dose of ARB for mice was 345.3 mg·kg−1·d−1, the inoculated mice received the following treatment: ARB at 180.0, 90.0, or 45.0 mg·kg−1·d−1, OSE at 22.5 mg·kg−1·d−1, 0.5% methylcellulose solution in the viral control group and the normal control group, respectively. The drugs were administered via oral gavage once a day for 5 d27.
Twelve mice per group were observed for mortality and weighed daily for 15 d after infection in the survival study. The protection was estimated by body weight evaluation, the reduction of mortality and prolongation of median time to death (MTD)28.
Another 12 mice from each group were sacrificed on d 5 after viral exposure. Lung tissues were harvested and weighed. The lung index was expressed as the ratio of mean lung weights to mean body weights. The collected lung samples were then divided into three subgroups based on lung index. One subgroup was subsequently homogenized to 10% (w/v) suspensions in test medium. The homogenates were frozen and thawed twice to release the virus and centrifuged at 2000×g for 10 min. Virus titration was determined by plaque assay. Organs from another subgroup were used for pathological examination (H&E staining). Tissues from the last subgroup were used for RNA detection by real-time RT-PCR. Additional mice (4 mice/group) were managed the same as above, and the bronchoalveolar lavage fluid (BALF) samples (0.8 mL/mice) were collected. After centrifugation at 1000×g for 5 min, BALF supernatants were collected and stored at −20 °C until ELISA was performed29.
For the time-of-addition-effect of ARB on the inflammation induced by IFV, additional mice from ARB treatment group, mock-infected group (treated with 90 mg·kg−1·d−1 ARB) and control group were sacrificed at 1, 3, and 5 d after exposure (4 mice/group per day). Lung tissues were collected for real-time RT-PCR to assess the cytokine transcriptional levels.
To determine the effect of ARB on the acute inflammation induced by poly I:C, the mice (4 mice/group) were pretreated with 0.5% methylcellulose solution or ARB via oral gavage once a day for 2 d. Two hours after the last administration, mice were given an intraperitoneal injection of PBS or poly I:C (100 μg/mouse)30. After another 4 h, mice were sacrificed and the sera were isolated and stored at −20 °C until ELISA was performed.
Ex vivo peritoneal macrophage infection and treatment
Murine peritoneal macrophages were isolated and cultivated as described previously31. Cultures were challenged with 2.0 MOI of influenza A/FM/1/47 (mouse adapted) virus or 20 μg/mL Poly I:C. After 1 h of adsorption or 30 min of activation, the cells were washed twice with PBS and further incubated with different doses of ARB (20, 10, and 5 μmol/L) diluted in the maintaining medium. The mock-infected group treated with 20 μmol/L of ARB was also included. Cells were collected at 1, 3, and 6 h after challenge. Transcriptional profiling of the viral-induced cytokine response was determined by real-time PCR. Supernatants from the groups of ARB (20 μmol/L), mock control, viral control, poly I:C control and normal control mice at 6 h were collected for ELISA analysis.
Plaque reduction assay
The titers of infectious viral particles from lung homogenate were determined by the standard plaque formation assay with minor modifications32. Replicate aliquots (500 μL/well) of serial 10-fold dilutions were inoculated onto MDCK cell monolayers in 6-well plates and incubated at 35 °C for 1 h to determine viral adsorption. After removal of the excess virus medium and washing three times with PBS, the cells were covered with media containing 1.2% agarose and 2 μg/mL trypsin. The agarose overlay was removed after incubation for 48 h. The cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet. Finally, plaques were counted and viral titers (PFU/mL) were determined.
Real-time RT-PCR assay
Total RNA was extracted from samples using an EZNA Total RNA Kit (OMEGA) according to manufacturer's instructions. For first-strand cDNA synthesis, 1 μg of total RNA was primed with random primers by Reverse Transcriptase (Promega). Then, 1/20 volume of cDNA was amplified on a Bio-Rad CFX96 instrument using SYBR Green Real-time PCR Master Mix Reagent (Toyobo). The reaction was performed at 95 °C for 3 min; 40 cycles of 95 °C for 10 s, 55 °C for 10 s, 72 °C for 10 s, followed by melting curve analysis. The amplification was performed using the following primer sets (Table 1). All the primers were from the RTPrimer Database at http://www.rtprimerdb.org/. The level of gene transcription was determined by comparing data from different treatment groups to the normal control group based on the comparative ΔΔCT method.
ELISA assay
The secreted inflammatory cytokines (IL-1β, IL-6, IL-12p70, IL-10, and TNF-α) in different samples (BALF, serum and supernatant) were tested simultaneously by ELISA kits (Dakewe). The experiment was performed according to the manufacturer's instructions.
Statistical analysis
Data are presented as the mean±SD. All the data were analyzed by SPSS 17.0 software. Weight loss data were checked by repeated measures and a mixed model multivariate analysis of variance process. Statistic differences between groups were determined by one-way ANOVA with Bonferroni's multiple comparison tests or two-way ANOVA with Bonferroni's post-tests. The probability of the mouse survival was estimated by Kaplan-Meier method and further analyzed by Log Rank pairwise tests over strata.
Results
ARB inhibited IFV A (H1N1) infections in vitro
To investigate the inhibitory effects of ARB on A/FM/1/47 and A/Hubei/74/2009, three different modes of treatment were tested as described in Materials and methods. As shown in Table 2, both seasonal and pandemic H1N1 influenza virus were equally sensitive to ARB, even 1 h after virus adsorption (EC50: 17.2 μmol/L for A/FM/1/47 and 18.2 μmol/L for A/Hubei/74/2009), and the inhibitory efficacy was compatible to the other two modes: the simultaneous treatment mode and pre-treatment mode (anti-virus adsorption and penetration). The efficacy of ARB in blocking the early stage of the two H1N1 strains was consistent with other reports that have identified with therapeutic indices (TI) from 3.1 to 3.612,21.
ARB alleviates the clinical signs caused by IFV A (H1N1) infection in mice
To further investigate whether ARB was active against H1N1 influenza in vivo when administered after viral infection, mice infected with the A/FM/1/47 H1N1 (mouse adapted) virus, which developed fatal viral pneumonia, were used as an evaluation model. Body weight loss, lethality, median survival time and viral titers in the lungs were employed to determine the antiviral efficiency of ARB.
As shown in Figure 1A, the virus-infected mice exhibited a tendency toward weight loss from the 3rd day post-infection (dpi). However, the weight loss was suppressed with treatment by ARB in 180.0 and 90.0 mg·kg−1·d−1 (Figure 1A). The survival curve further confirmed the efficacy of ARB against lethal influenza infection. As shown in Figure 1B, the survival rate and survival time of the ARB-treated groups at dosages of 180.0 and 90.0 mg·kg−1·d−1 were higher or longer than those of the viral control group (P<0.01). Moreover, ARB treatment reduced viral replication in a dose-dependent manner (Figure 1C), as measured by viral titers in the murine lungs at 5 dpi, which indicated that the lethal IFV was sensitive to post-treatment with ARB in vivo. Taken together, post-treatment with ARB can effectively reduce the lethality, body weight loss, viral titers and reduction of survival time in A/FM/1/47 H1N1 infected mice, and the efficacy of ARB treatment in alleviating clinical signs was comparable to OSE treatment, except for reducing viral titer.
ARB alleviated IFV A (H1N1)-induced clinical signs in mice. BALB/c mice (n=12 mice/group) were infected with the Influenza A/FM/1/47 (H1N1) virus (10 LD50 per mouse, in). After 4 h, mice were treated with 0.5% methylcellulose solution (normal control, NC; viral control, VC), ARB (180, 90, or 45 mg·kg−1·d−1), or OSE (oseltamivir, 22.5 mg·kg−1·d−1) qd for 5 d, respectively. Body weight (A) and lethality (B) were collected daily for 15 d. Viral titers of lungs (C) at 5 dpi were determined by plaque assay. cP<0.01.
ARB decreased the severity of viral lung lesions
Pathological examination (Figure 2B) showed that ARB treatment attenuated the virus-induced thickening of pulmonary alveoli walls and that the infiltration of inflammatory cells into interstitial septa as the drug dose increased. The analysis of the mean lung index further confirmed that the indices of groups treated with 180.0 and 90.0 mg·kg−1·d−1 of ARB were 24% and 37% higher than the normal control, while the index of viral control group was twice as high as that of the normal control group (Figure 2A). It should be noted that the lung indices of the 22.5 mg·kg−1·d−1 OSE treatment was 40% higher than the normal control, while the viral titer of this group was 6.3 or 31.6 lower than those of the 180.0 or 90.0 mg·kg−1·d−1 ARB-treated groups (Figure 1C). Thus, ARB appeared to be more efficient at reducing viral lung lesions compared to OSE treatment. Regarding the inflammatory cytokine production, the cytokine levels in the BALF that was collected from the murine lung at the 5th dpi were reduced by ARB, except for the anti-inflammatory cytokine IL-10 (Figure 2C). ARB was more effective than OSE at modulating the virus-associated inflammatory cytokines. Thus, post-treatment with ARB can effectively alleviate the severity of fatal viral pneumonia through the inhibition of the inflammatory response.
ARB reduced IFV A (H1N1)-induced lung lesion in mice. BALB/c mice (n=12 mice/group) were infected with Influenza A/FM/1/47 (H1N1) virus (10 LD50 per mouse, in). After 4 h, mice were treated with 0.5% methylcellulose solution (normal control, NC; viral control, VC), ARB (180, 90, or 45 mg·kg−1·d−1) or OSE (O, 22.5 mg·kg−1·d−1) qd for 5 d. All the mice were sacrificed at the 5th dpi. (A) Lung index of each group. The index was determined as lung weight/final body weight (LW/BW). (B) Pathological examination for each group. (C) Cytokine profile in BALF of mice. bP<0.05, cP<0.01. UD=under detectable level.
ARB suppressed the transcription of IFV-associated inflammatory cytokines in vivo
To further confirm whether cytokine production during treatment was affected by ARB, a time-course study for the transcription of cytokines was performed. As shown in Figure 3, seven cytokines induced by influenza were down-regulated by post-treatment with ARB, and five of them (IL-1β, IL-6, IL-12p40, TNF-α, and IFN-β) varied significantly compared with the viral control group (P<0.05). The mRNA level of IL-6 was dramatically diminished by ARB in a dose-dependent manner since the 1st dpi. The transcription of TNF-α, IL-12p40, and IL-1β decreased similarly to IL-6. In contrast to other cytokines, the mRNA level of IL-10 was significantly elevated by ARB since the 3rd dpi. The expressions of IFN-α and IFN-γ were not significantly affected by ARB, but IFN-β1 was reduced at the 5th dpi in the murine lung. Therefore, post-treatment with ARB can control the severity of virus-associated inflammation through suppressing the transcriptions of IL-1β, IL-6, IL-12p40, and TNF-α and elevating the transcription of the anti-inflammatory cytokine IL-10.
ARB decreased the transcription of cytokines in murine lung following the IFV A (H1N1) Infection. BALB/c mice (n=12 mice/group) were infected with influenza A/FM/1/47 (H1N1) virus (10 LD50 per mouse, in) or PBS. After 4 h, mice were treated with 0.5% methylcellulose solution (normal control, NC; viral control, VC) or ARB (180, 90, or 45 mg·kg−1·d−1) qd for 5 d. Mock infected group was treated with 90 mg·kg−1·d−1 of ARB. The mice were scheduled for sacrifice at 1, 3, and 5 dpi. Lungs of the mice were collected and homogenated. Real-time PCR analysis was used to determine the mRNA expression level of cytokines normalized to cellular GAPDH. bP<0.05, cP<0.01.
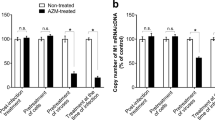
ARB suppressed the inflammatory cytokine response in peritoneal macrophages
As macrophages play a central role in both influenza-induced lung lesions and the host's cytokine-mediated response3, we examined whether ARB can inhibit the expression of inflammatory cytokines in macrophages at the early stage of viral infection. The stimulation was managed by IFV or a mimic of the double viral RNA, poly I:C. As shown in Figure 4A and 4B, the up-regulation of TNF-α, IL-1β, and IL-6 mRNA was significantly suppressed by ARB in a dose-dependent manner after 4 hpi. With ARB treatment, the level of IL-10 mRNA was much higher than that of the viral control or the poly I:C control at 6 hpi. The effect of ARB on IL-12 and interferon was not obvious (data not shown). As determined by ELISA, the secreted cytokines in the supernatant also exhibited a similar tendency (Figure 4C and 4D). Taken together, these data indicate that ARB can efficiently suppress the inflammatory cytokine response that is mediated by macrophages at the early stage of viral infection.
ARB suppressed the acute inflammation in peritoneal macrophage following the IFV A (H1N1) Infection or poly I:C. Murine peritoneal macrophage were isolated and cultured as the protocol of Zhang et al31. Within 24 h the cells were stimulated with 2 MOI of influenza A/FM/1/47 (H1N1) for 1 h or 20 μg/mL poly I:C for 30 min. The cells were then treated with ARB (20, 10, or 5 μmol/L) or free serum medium (normal control, NC; viral control, VC). Mock infected group (M) was treated with 20 μmol/L of ARB. Cells were collected at 2, 4, or 6 h post activation. Transcription for cytokine response was used to determine the mRNA expression level of cytokines normalized to cellular GAPDH by RT-PCR analysis. Supernatants from the groups of ARB (20 μmol/L), mock, positive control and normal control at the point of 6 h were collected for ELISA assay. (A and C: Influenza challenged; B and D: poly I:C challenged). bP<0.05, cP<0.01. UD=under detectable level.
ARB suppressed poly I:C-induced acute inflammation in vivo
The above results led us to further investigate whether ARB modulates the inflammatory cytokine response induced by poly I:C in vivo. As shown in Figure 5, a single intraperitoneal injection of poly I:C (100 μg/mouse) increased the serum levels of all five cytokines 4 h later. Administration of ARB prior to injection with poly I:C led to a marked suppression of TNF-α, IL-6, and IL-12p70, while the production of IL-10 was increased. These data suggested that ARB can suppress the inflammatory cytokine response induced by poly I:C.
ARB modulates the production of cytokine in the serum of poly I:C stimulated mice. Mice (n=4 mice/group) were treated with 0.5% methylcellulose solution (normal control, NC; viral control, VC) or ARB via gavage once a day for 2 d. Two hours after the last administration, mice were given an intraperitoneal injection of PBS or poly I:C (pIC, 100 μg/mouse). Mock infected group (M) was treated with 90 mg·kg−1·d−1 of ARB. After another 4 h, mice were sacrificed, serum was isolated and cytokines were tested by ELISA. bP<0.05. UD=under detectable level.
Discussion
ARB has been widely used for almost 20 years in Russia. However, the mechanism of ARB's action is complex21,22,23,24,3334. As ARB can decrease the severity of the disease, improve the catarrhal syndrome and reduce the risk of complications in patients22, we suspected that ARB might also have anti-inflammatory activity in addition to its antiviral effect. Here, we demonstrated that ARB could not only inhibit both seasonal and pandemic influenza infection in vitro and in vivo, but it can also alleviate the severity of the disease through suppressing the inflammation induced by lethal influenza in murine lungs. Because macrophages play a central role in response to infection, we also determined the effect of ARB on peritoneal macrophages exposed to IFV and a virus-like mimic, poly I:C. Our results confirmed that ARB could diminish the acute inflammation induced by IFV or poly I:C through modulating the excessive cytokine response produced by macrophages. These data give support for ARB's possible use against the cytokine dysregulation in viral infection.
The broad-spectrum antiviral activity of ARB suggests that it may be unlikely to target specific viral proteins or viral receptors, but that it targets some common steps of virus infection10,35. Previous studies have reported that ARB inhibited influenza growth by blocking the fusion between the viral envelope and the endosomal membrane19. Thus, the delivery of ARB in those studies was usually performed before viral infection. In our study, the EC50 of the pre-treatment mode for pandemic influenza A/Hubei/74/2009 (H1N1) and seasonal influenza A/FM/1/47 (H1N1) were 18.3 μmol/L and 19.4 μmol/L, respectively, which are consistent with previous studies12,21,35. However, the anti-fusion mechanism could not explain the sensitivity to ARB of non-enveloped viruses, such as Coxsachievirus B5 and Poliovirus 114,21. Our study also showed that ARB could efficiently inhibit influenza infections when given after virus infection both in vitro and in vivo. It should be noted that post-treatment with ARB inhibited only 20% of viral reproduction during a one-cycle infection experiment as tested by fluorescence or EMIT33. In our experiment, post-treatment with the same concentration of ARB reduced the CPE by approximately 50% at 72 hpi. It seems that post-treatment with ARB was more likely to protect cells from death rather than having a direct antiviral effect. However, there might be an alternative explanation. Some inflammatory inhibitors, such as acetylsalicylic acid, do not affect the accumulation of the viral RNA or proteins during a one-cycle infection, but these inhibitors can lead to the retention of vRNPs in the nucleus, which subsequently inhibits the propagation of progeny virus36. Thus, it cannot be ruled out that ARB has the same potential if it has a similar anti-inflammatory profile.
The effect of ARB on the transcription of interferon was also investigated in this study. The results indicated that the transcription of IFN-β was significantly decreased in murine lungs at 5th dpi (Figure 3). The gene expression of IFN-α and IFN-γ were not significantly decreased by ARB during the entire period of observation (Figure 3). Our data support the results of Boriskin et al, who noted that ARB does not play a role in the induction of interferon24. Furthermore, other studies have also indirectly indicated that the antiviral activity of ARB may not depend on interferon induction, as ARB could efficiently inhibit influenza virus and hantavirus in Vero and Vero E6 cells that lack the IFN gene15,21.
The virus-induced cytokine response contributes to the activation of the immune system and the damage to the host4,37. Suppression of these cytokines can potentially control the severity of the virus-induced inflammatory complications and finally lower the mortality4,37. Interestingly, the inflammatory cytokines IL-1β, IL-6, IL-12, and TNF-α were diminished in response to ARB in IFV-infected murine lungs (Figure 2C). The transcription of IL-6 was dramatically reduced from the 1st dpi (Figure 3). Furthermore, IL-10, an anti-inflammatory cytokine, was promoted by ARB. It should be noted that the level of IL-10 in the mock group was not elevated by ARB (Figure 5), suggesting that ARB did not exert its modulating effects when used alone and that additional stimulation is needed. The correlation between inflammatory cytokines and influenza pathogenicity has been well demonstrated38,39. The expression of inflammatory cytokines not only activates the immune response to the virus but also damages the host4. The reduction in IL-1β, IL-6, IL-12, and TNF-α levels promoted by ARB should play an important role in reducing stress due to the immune system, preventing tissue damage and improving the prognosis of infected patients. This hypothesis was confirmed by pathological examination and lung index evaluation, which found that post-treatment with ARB alleviated the severity of virus-associated pneumonia (Figure 2) and the lethality and the reduced median survival time for mice (Figure 1). The lethal lung pathology caused by IFV was due to the excessive cytokine response that was primarily produced by the activated macrophages4. IFV-infected peritoneal macrophages can produce IL-1β, IL-6, IL-10, TNF-α, and minimal IL-12 even in early phases of infection and can produce interferon 48 h after stimulation40,41,42. In our study, ARB diminished the inflammatory cytokines induced by IFV or the virus-like mimic poly I:C in peritoneal macrophages (Figure 4 and 5), which confirmed that ARB can also inhibit the inflammatory cytokine response that is mediated by macrophages.
The activation of inflammatory cytokines and virus replication share the same intercellular pathway43,44,45. The fact that ARB could inhibit both the replication of the virus and the expression of inflammatory cytokines might provide some clues for understanding the mechanism by ARB acts on these cascades. Further studies of these pathways using microarray analysis and Western blotting analysis will be needed. Collectively, ARB can efficiently inhibit both seasonal influenza A/FM/1/47 (H1N1) and pandemic influenza A/Hubei/74/2009 (H1N1) infections. Both its antiviral effect and its modulatory function on virus-induced inflammatory cytokines, including IL-1β, IL-6, IL-10, IL-12, and TNF-α, might contributed to the beneficial effect of the drug. This study provides evidence for the therapeutic use of ARB for H1N1 infections and for the potential use of this drug as an anti-inflammatory agent against virus-induced cytokine dysregulation.
Author contribution
Qiang LIU, Hai-rong XIONG, and Zhan-qiu YANG designed the research plan; Qiang LIU, Hai-rong XIONG, Li LU, Yuan-yuan LIU, and Fan LUO performed the research; Qiang LIU, Hai-rong XIONG, and Wei HOU analyzed the data; and Qiang LIU, Hai-rong XIONG, Li LU, and Zhan-qiu YANG wrote the paper.
References
Clem A, Galwankar S . Seasonal influenza: waiting for the next pandemic. J Glob Infect Dis 2009; 1: 51–6.
Fields BN, Knipe DM, Howley PM . Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
La Gruta NL, Kedzierska K, Stambas J, Doherty PC . A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol 2007; 85: 85–92.
Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005; 310: 77–80.
CDC. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 433–5.
Cheng PK, Leung TW, Ho EC, Leung PC, Ng AY, Lai MY, et al. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1). Emerg Infect Dis 2009; 15: 966–8.
Zaraket H, Saito R, Suzuki Y, Baranovich T, Dapat C, Caperig-Dapat I, et al. Genetic makeup of amantadine-resistant and oseltamivir-resistant human influenza A/H1N1 viruses. J Clin Microbiol 2010; 48: 1085–92.
Ludwig S . Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J Antimicrob Chemother 2009; 64: 1–4.
Josset L, Textoris J, Loriod B, Ferraris O, Moules V, Lina B, et al. Gene expression signature-based screening identifies new broadly effective influenza A antivirals. PLoS One 2010; 5: e13169.
Leneva IA, Fediakina IT, Gus'kova TA, Glushkov RG . Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A. Ter Arkh 2005; 77: 84–8.
Leneva IA, Shuster AM . Antiviral etiotropic chemicals: efficacy against influenza A viruses a subtype H5N1. Vopr Virusol 2006; 51: 4–7.
Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol 2007; 152: 1447–55.
Leneva IA, Sokolova MV, Fediakina IT, Khristova ML, Fadeeva NI, Gus'kova TA . Study of the effect of antiviral drugs on the reproduction of the respiratory syncytial virus by enzyme immunoassay. Vopr Virusol 2002; 47: 42–5.
Zhong Q, Yang Z, Liu Y, Deng H, Xiao H, Shi L, et al. Antiviral activity of Arbidol against Coxsackie virus B5 in vitro and in vivo. Arch Virol 2009; 154: 601–7.
Deng HY, Luo F, Shi LQ, Zhong Q, Liu YJ, Yang ZQ . Efficacy of arbidol on lethal hantaan virus infections in suckling mice and in vitro. Acta Pharmacol Sin 2009; 30: 1015–24.
Chai H, Zhao Y, Zhao C, Gong P . Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem 2006; 14: 911–7.
Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ . Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 2008; 15: 997–1005.
Brooks MJ, Sasadeusz JJ, Tannock GA . Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr Opin Pulm Med 2004; 10: 197–203.
Teissier E, Zandomeneghi G, Loquet A, Lavillette D, Lavergne JP, Montserret R, et al. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One 2011; 6: e15874.
Leneva IA, Russell RJ, Boriskin YS, Hay AJ . Characteristics of arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol. Antivir Res 2009; 81: 132–40.
Brooks MJ, Burtseva EI, Ellery PJ, Marsh GA, Lew AM, Slepushkin AN, et al. Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions. J Med Virol 2012; 84: 170–81.
Glushkov RG, Gus'kova TA, Krylova L, Nikolaeva IS . Mechanisms of arbidole's immunomodulating action. Vestn Ross Akad Med Nauk 1999; (3): 36–40.
Silin DS, Lyubomska OV, Ershov FI, Frolov VM, Kutsyna GA . Synthetic and natural immunomodulators acting as interferon inducers. Curr Pharm Design 2009; 15: 1238–47.
Boriskin YS, Pecheur EI, Polyak SJ . Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J 2006; 3: 56.
Zhao JR, Li YD, Pan LM, Zhu N, Ni HX, Xu GZ, et al. Genetic characteristics of 2009 pandemic H1N1 influenza A viruses isolated from Mainland China. Virol Sin 2011; 26: 418–27.
Xu L, Bao L, Li F, Lv Q, Ma Y, Zhou J, et al. Adaption of seasonal H1N1 influenza virus in mice. PLoS One 2011; 6: e28901.
Drusano GL, Preston SL, Smee D, Bush K, Bailey K, Sidwell RW . Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing. Antimicrob Agents Chemother 2001; 45: 2115–8.
Triana-Baltzer GB, Gubareva LV, Nicholls JM, Pearce MB, Mishin VP, Belser JA, et al. Novel pandemic influenza A (H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One 2009; 4: e7788.
Yatmaz S, Seow HJ, Gualano RC, Wong ZX, Stambas J, Selemidis S, et al. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am J Respir Cell Mol Biol 2013; 48: 17–26.
De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest 2010; 120: 2395–405.
Zhang X, Goncalves R, Mosser DM . The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008; Chapter 14: Unit 14.1.
Prichard MN, Turk SR, Coleman LA, Engelhardt SL, Shipman C Jr, Drach JC . A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J Virol Methods 1990; 28: 101–6.
Leneva IA, Fadeeva NI, Fedyakina IT, Gus'kova TA, Khristova NL, Sokolova MV, et al. Use of enzyme immunoassay to identify virus-specific antigens in studying a new anti-influenza preparation, arbidol. Pharm Chem J 1994; 28: 506–605.
Glushkov RG, Fadeeva NI, Leneva IA, Gerasina SF, Budanova LI, Sokolova ND, et al. Molecular biological characteristics of the action of arbidol — A new antiviral drug. Pharm Chem J 1992; 26: 106–15.
Leneva IA, Fediakina IT, Eropkin M, Gudova NV, Romanovskaia AA, Danilenko DM, et al. Study of the antiviral activity of Russian anti-influenza agents in cell culture and animal models. Vopr Virusol 2010; 55: 19–27.
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, et al. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol 2007; 9: 1683–94.
Sladkova T, Kostolansky F . The role of cytokines in the immune response to influenza A virus infection. Acta Virol 2006; 50: 151–62.
Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC . Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol 2010; 84: 7569–80.
Pommerenke C, Wilk E, Srivastava B, Schulze A, Novoselova N, Geffers R, et al. Global transcriptome analysis in influenza-infected mouse lungs reveals the kinetics of innate and adaptive host immune responses. PLoS One 2012; 7: e41169.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 2002; 3: 196–200.
Murphy EA, Davis JM, McClellan JL, Carmichael MD, Rooijen NV, Gangemi JD . Susceptibility to infection and inflammatory response following influenza virus (H1N1, A/PR/8/34) challenge: role of macrophages. J Interferon Cytokine Res 2011; 31: 501–8.
Osterlund P, Pirhonen J, Ikonen N, Ronkko E, Strengell M, Makela SM, et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J Virol 2010; 84: 1414–22.
Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S . Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev 2001; 12: 171–80.
Nimmerjahn F, Dudziak D, Dirmeier U, Hobom G, Riedel A, Schlee M, et al. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J Gen Virol 2004; 85: 2347–56.
Matikainen S, Pirhonen J, Miettinen M, Lehtonen A, Govenius-Vintola C, Sareneva T, et al. Influenza A and sendai viruses induce differential chemokine gene expression and transcription factor activation in human macrophages. Virology 2000; 276: 138–47.
Acknowledgements
This work was supported by the National Mega Project on Major Drug Development (2009ZX09301-014-1), the National Natural Science Foundation of China (No 30873104 and 81000734) and the Fundamental Research Funds for the Central Universities (4101045).
We thank Prof Tian-xian LI for the viruses. We gratefully acknowledge all individuals within the ABSL-3 team for their kindness and their technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Xiong, Hr., Lu, L. et al. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin 34, 1075–1083 (2013). https://doi.org/10.1038/aps.2013.54
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.54
Keywords
This article is cited by
-
Investigational antiviral drugs for the treatment of COVID-19 patients
Archives of Virology (2022)
-
The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro
Cell Discovery (2020)
-
The Antiviral Drug Arbidol Inhibits Zika Virus
Scientific Reports (2018)
-
Identification of Inhibitory Compounds Against Singapore Grouper Iridovirus Infection by Cell Viability-Based Screening Assay and Droplet Digital PCR
Marine Biotechnology (2018)
-
The cytokine storm of severe influenza and development of immunomodulatory therapy
Cellular & Molecular Immunology (2016)