Abstract
Aim:
To investigate the effects of salvianolate, a water-soluble active compound from Salvia miltiorrhiza Bunge, on reactive oxygen species (ROS) production in mouse cardiomyocytes in vitro.
Methods:
Primary ventricular cardiomyocytes were prepared from neonatal mouse. The cell viability was determined using MTT assay. Culture medium for each treatment was collected for measuring the levels of NO, iNOS, total antioxidant capacity (TAOC) and transforming growth factor β1 (TGFβ1). TGFβ1 and Smad2/3 expression in the cells was detected with Western blotting.
Results:
H2O2 (1.25 mmol/L) did not significantly affect the cell viability, whereas the high concentration of salvianolate (5 g/L) alone dramatically suppressed the cell viability. Treatment of the cells with H2O2 (1.25 mmol/L) markedly increased ROS and iNOS production, and decreased the levels of NO, TAOC and TGFβ1 in the culture medium. Furthermore, the H2O2 treatment significantly increased TGFβ1 and Smad2/3 expression in the cells. Addition of salvianolate (0.05, 0.1, and 0.5 g/L) concentration-dependently reversed the H2O2-induced alterations in the culture medium; addition of salvianolate (0.05 g/L) reversed the H2O2-induced increases of TGFβ1 and Smad2/3 expression in the cells. Blockage of TGFβ1 with its antibody (1 mg/L) abolished the above mentioned effects of salvianolate.
Conclusion:
Salvianolate inhibits ROS and iNOS production and increases TAOC and NO levels in H2O2-treated cardiomyocytes in vitro via downregulation of Smad2/3 and TGFβ1 expression. High concentration of salvianolate causes cytotoxicity in mouse cardiomyocytes.
Similar content being viewed by others
Introduction
Congestive heart failure (CHF) is a major cause of cardiac morbidity and mortality world-wide. Recent experimental and clinical studies have suggested that oxidative stress plays an important role in myocardial remodeling and heart failure1,2. The increased production of oxygen radicals in the failing heart has been linked to the development of left ventricular hypertrophy and heart failure progression. Reactive oxygen species (ROS) cause contractile failure and structural damage in the myocardium. The importance of oxidative stress is emerging with respect to a pathophysiological mechanism of LV remodeling responsible for heart failure progression.
Transforming growth factor beta (TGFβ) is an important fibrogenic growth factor. TGFβ can induce renal extracellular matrix (ECM) accumulation and organ fibrosis, including cardiac fibrosis in chronic heart failure patients. ROS is a signaling molecule that mediates the effects of TGFβ1. TGFβ1 induces prolonged mitochondrial ROS generation in Mv1Lu cells3. Elevated systemic TGFβ impairs aortic vasomotor function through the activation of NADPH oxidase-driven superoxide production and leads to hypertension, myocardial remodeling, and increased plaque formation in apoE(−/−) mouse4.
Salvianolate is a newly discovered water-soluble phenolic compound that is one of the most bioactive compounds in Salvia miltiorrhiza Bunge. As a highly purified aqueous extract from Danshen, salvianolate contains mainly magnesium lithospermate B (≥85%), rosmarinic acid (≥10.1%) and lithospermic acid5,6. Salvianolate inhibits cytokine gene expression in the small intestine of cirrhotic rats5. Salvianolate can inhibit cardiomyocytic apoptosis and improve heart function after acute myocardial infarction (AMI) in swine7.
To date, the effect of salvianolate on ROS production in cardiomyocytes remains unknown. In this study, we aimed to investigate whether salvianolate influences ROS production in cardiomyocytes undergoing H2O2-induced oxidative damage. We also explored the role of the TGF signaling pathway in the regulation of H2O2-induced ROS injury and whether this pathway is involved in the protective effects of salvianolate.
Materials and methods
Neonatal mouse primary cardiomyocyte culture
The procedure used to culture ventricular cardiomyocytes from neonatal mouse was established by modifying previously described methods6. Briefly, one- to three-day-old neonatal mice were euthanized by cervical dislocation. Hearts were removed aseptically from the mouse, and the ventricles were retained and kept in Hanks' balanced salt solution. The cells were dissociated at 37 °C for 8 min in an enzyme solution. The suspended cells were then collected and plated at a density of 1.0×105 cells/cm2 and incubated under the same conditions as described above. The H2O2-treated cardiomyocytes were divided into 5 groups, namely, the control, 0.05 g/L salvianolate-treated, 0.1 g/L salvianolate-treated, 0.5 g/L salvianolate-treated and TGFβ1 inhibitor groups. Cardiomyocytes in all H2O2-treated groups were treated with 1.25 mmol/L H2O2. Cardiomyocytes in the TGFβ1 inhibitor group were administered TGFβ1 neutralization antibody (1 mg/L, Biolegend, CA, USA) together with salvianolate and H2O2. The non-treated cardiomyocyte group was used as a control. All cells were cultured in a 5% CO2 incubator for 24 h before analyses.
MTT assay
Cell viability was determined by a short-term microculture MTT assay. Cardiomyocytes were plated at a density of 3×104 cells/well on 96-well microplates containing 150 mL FBS-DMEM, and four replications were performed of each treatment to reduce lab errors. After being cultured for 48 h, the cells were exposed to different concentrations (0.075, 0.15, 0.3, and 0.6 mmol/L) of H2O2 for 3 h, and the media was then replaced by 100 mL of DMEM and 20 μL of MTT solution (5 mg/mL). The cells were incubated for another 4 h. After incubation, the media and MTT solution were removed. The remaining formazan blue crystals were dissolved with DMSO. Absorbance at 570 nm was measured by a Multiskan MK3 plate reader (Thermo Lab Systems).
Measurement of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) levels in culture medium
Culture medium for each treatment was collected from triplicate wells to reduce system error and used to detect NO and iNOS levels. The nitrite level in cell-free medium was measured using Griess reagent as a reflection of NO production. The NO level was assessed by measuring the level of nitrite (NO2) using a spectrophotometer set to read the absorbance at a wavelength of 550 nm. Nitrite concentrations were calculated using a sodium nitrite standard. Fresh culture medium served as a blank in all experiments. The iNOS level was measured using a chemical colorimetric method according to the NOS reagent kit instructions. One hundred microliter of culture medium was applied to assay NOS activity, with absorbance readings taken at 550 nm.
Total antioxidant capacity (TAOC) of culture medium
TAOC was measured in serum taken from triplicate wells to reduce system error for each treatment using a commercial kit (Randox Laboratories, Antrim, UK), and the assay results were expressed as Trolox equivalents (mmol/L). RBC superoxide dismutase (SOD) and whole blood Gpx activities were determined using Randox kits (Antrim, UK), and the enzyme activities were expressed as U/mL. Serum vitamin C levels were assayed spectrophotometrically using the 2,4-dinitrophenylhydrazine method (normal range: 0.5–1.5 mg/dL).
Measurement of TGFβ1 level in cell culture medium
To determine the extracellular TGFβ1 level, 100 μL of culture medium for each treatment was obtained from triplicate wells to reduce system error, aiming to simulate the intravascular environment with culture medium. TGFβ ELISA kits were purchased from Abcam (Cat ab119557, Hong Kong, China), and the experiments were carried out in accordance with the protocol provided.
Western blotting for TGFβ1 and Smad2/3
We used Western blot assays to evaluate the protein expression level of TGFβ1 and Smad2/3. Assays were performed in triplicate to reduce experimental error. Cells were lysed using lysis buffer (10 mmol/L Tris, 1 mmol/L EDTA, 1% Triton X-100, 1 mmol/L Na3VO4, 1 mmol/L AEBSF, 0.3 μmol/L aprotinin, 10 μmol/L bestatin, 10 μmol/L E64, and 100 μmol/L leupeptin); 250 μg of crude protein lysate was resolved on 10% SDS-PAGE gels. After protein transfer to nitrocellulose paper, the blots were probed with a 1:1000 (v/v) dilution of polyclonal anti-TGFβ1 and Smad2/3 primary antibody. After hybridization at 37 °C, the blots were washed and hybridized with a 1:2000 dilution of goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, USA). Blocking was performed with 5% skim milk in t-TBS buffer. A signal was generated by adding enhanced chemiluminescent reagent.
Statistical analysis
All of the data in the different experimental groups were expressed as the mean±SD. Differences between the groups were assessed using one-way ANOVA and the t-test. P<0.05 was considered to be statistically significant.
Results
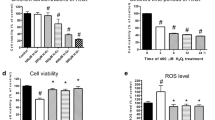
Effect of salvianolate on cell viability as evaluated by MTT assays
Four repeated wells in each group were tested with an MTT assay; triplication was performed for the entire experiment, and the mean cell viability rates were recorded. Contrary to expectation, the cell viability of H2O2-treated cardiomyocytes was not significantly reduced (control, 100%±8.49%; H2O2, 99%±1.69%). However, both the cell viability of the salvianolate-treated groups and the inhibitor group appeared significantly different from the control in both H2O2-treated groups and non-treated groups. Notably, with increasing salvianolate concentration, cell viability decreased, indicating the potential toxicity of high-concentration salvianolate (Figure 1).
Effect of salvianolate on cell viability of neonatal mouse primary cardiomyocytes. bP<0.05, cP<0.01 compared with control group. fP<0.01, paired sample T test, compared with paired H2O2-treated groups. Mean±SD.
NO and iNOS levels in cardiomyocyte groups
H2O2 administration significantly reduced NO levels in cardiomyocytes compared with controls (58.47±9.54 vs 26.37±9.1 μmol/L, P<0.001, Figure 2A). Salvianolate administration induced marked increases in NO level (26.37±9.1 vs 40.45±9.3 μmol/L, P=0.03). However, when TGFβ1 antibody was administrated, the NO level decreased to the H2O2-treated cell level (30.49±6.3 vs 26.37±9.1 μmol/L, P=0.034). With increasing salvianolate concentration, NO levels appeared to continuously increase (Figure 2A), while iNOS levels displayed the opposite trend. Compared with the activity of control cells, iNOS activity was markedly significantly decreased after H2O2 treatment (35.76±6.2 vs 17.57±5.8 U/mL, P<0.001, Figure 2B). Salvianolate administration induced a marked reduction in iNOS activity (35.76±6.2 vs 20.42±4.8 U/mL, P<0.001). TGFβ1 antibody administration caused an increase in iNOS activity (31.65±6.2 U/mL, P=0.012, Figure 2B).
NO and iNOS level in culture medium. (A) The NO level in culture medium of cardiomyocytes; (B) iNOS activity in culture medium; (C) ROS production in culture medium; (D) TAOC level in culture medium. bP<0.05, cP<0.01, compared with H2O2-treated group. Mean±SD.
ROS and TAOC level changes
ROS and TAOC measurements in cultured cardiomyocytes were repeated three times, and the mean ROS levels were recorded. In comparison with normal controls, the levels of ROS in H2O2-treated cells were significantly increased (247.40±8.45 vs 80.56±12.34 μmol/L, P<0.001). Likewise, the levels of ROS were significantly reduced in the salvianolate group when compared with the H2O2 group (247.40±8.45 vs 156.52±11.36 μmol/L, P=0.02, 0.05g/L salvianolate-treated). When TGFβ1 antibody was administered, ROS levels increased again (205.33±12.1 μmol/L, P=0.018, Figure 2C).
TAOC levels were significantly reduced in H2O2-treated cardiomyocytes, which was enhanced by salvianolate administration (3.94 U/mg protein in control, 2.32 U/mg protein in H2O2-treated cells and 3.17 U/mg protein in 0.05 g/L salvianolate-treated cells); however, when TGFβ1 was blocked, the increase in TAOC was not observed (Figure 2D).
TGFβ1 levels in cardiomyocyte culture medium
TGFβ1 levels in cardiomyocyte culture medium were determined with ELISA. Compared with controls, the levels of TGFβ1 in H2O2-treated cells were significantly decreased (0.839±0.059 vs 1.755±0.037 ng/L, P<0.001). TGFβ1 significantly increased in the salvianolate group (1.79±0.068 ng/L vs H2O2-treated cells, P<0.001, Figure 3). Unlike the other measured parameters, with increasing salvianolate concentration, TGFβ1 levels appeared to approach those of the H2O2-treated group instead of those of the control group.
TGFβ1 level in cell culture medium, determined by ELISA. bP<0.05, cP<0.01, compared with H2O2 treated group. Mean±SD.
Protein levels of Smad2/3 and TGFβ1 in cardiomyocytes determined by Western blotting
The expression of Smad2/3 was shown in Figure 4. H2O2 treatment significantly induced the expression of TGFβ1 and Smad2/3 protein levels. Treatment with salvianolate markedly reduced Smad2/3 and TGFβ1 expression. However, high salvianolate concentration (0.5 g/L) caused reversedly overexpression of Smad2/3 and TGFβ1 compared to the H2O2-treated group, indicating the potential toxicity of high concentrations of salvianolate (Figure 4).
Relative intracellular Smad2/3 and TGFβ1 level by Western blotting. (A) Raw data. (B) Relative quantity of Smad2/3, adjusted with β-actin. (C) Relative quantity of TGFβ1, adjusted with β-actin. bP<0.05, cP<0.01, compared with H2O2 treated group.
Discussion
In this study, we report that salvianolate can maintain or even improve cell viability under external stimulation. Additionally, salvianolate can reverse the adverse effects of H2O2 stimulation by inhibiting H2O2-mediated ROS and iNOS production, increasing the levels of TAOC and NO, and downregulating increased Smad2/3 and TGFβ1 expression. Notably, blockage of TGFβ1 with its antibody depletes the protective effect of salvianolate, suggesting that this effect is mediated by the TGFβ1 signaling pathway.
The chronic release of ROS has been recently linked to the development of left ventricular hypertrophy and heart failure progression8, and appears to derive from nonphagocytic NAD(P)H oxidase and mitochondria. The fibrosis, collagen deposition, and metalloproteinase activation involved in the remodeling of the failing myocardium are all dependent on ROS released during the phenotypic transformation of fibroblasts to myofibroblasts associated with the progression of end-stage heart failure8,9,10,11.
TGFβ1 stimulates the production of ROS in various types of nonphagocytic cells, such as endothelial cells, epithelial cells, smooth muscle cells, and fibroblasts12,13. The ROS pathway has been shown to play an important role in TGFβ1-induced fibronectin and PAI-1 upregulation in NRK-52E cells14. Our results show that TGFβ1 levels changed consistently with ROS production. More importantly, the inhibition of TGFβ1 by its antibody abolished the decrease of ROS, suggesting that the interaction between ROS and TGFβ1 was involved in the mechanism behind the effect of salvianolate on cardiomyocytes.
The beneficial effects of salvianolate have been reported previously. However, our replicable and compelling cell viability data indicate that high concentrations of salvianolate are potentially toxic to cells, although other data including ELISA, Western blotting, and cell culture medium detection did not provide any direct evidence that the TGFβ pathway was involved in these toxic effects.
Salvianolate, combined with other medications, reduced glutathione in the progression of chronic renal failure in patients with chronic kidney diseases. Salvianolate can reduce the extent of postoperative intestinal adhesions, decrease the expression of IL-1β and TNF-α and inhibit the hyperplasia of fibrous connective tissue15. Salvianolate can reduce endotoxin levels, restore intestinal mucosal injury, and inhibit the expression of TNF-α and IL-6 in the small intestine of cirrhotic rats5. Salvianolate administered via intravenous drip can inhibit cardiomyocytic apoptosis and improve heart function AMI in pigs7. The facilitation of the migration of endothelial cells induced by monocytes by salvianolate has also been observed. Salvianolate stimulates the expression of VEGF and bFGF and their mRNA in monocytes and may induce endothelial cell migration via these two factors16. Salvianolate inhibits proliferation and endothelian release in cultured rat mesangial cells17. In this study, we reported that salvianolate may inhibit ROS production and increase the antioxidant capacity of cardiomyocytes, suggesting its potential in the treatment of chronic cardiac fibrosis.
References
Sorescu D, Griendling KK . Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 2002; 8: 132–40.
Guzman Mentesana G, Baez A, Cordoba R, Dominguez R, Lo Presti S, Rivarola W, et al. Role of mitochondria and reactive oxygen species in the progression of heart failure. Rev Fac Cien Med Univ Nac Cordoba 2010; 67: 150–8.
Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G . TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 2005; 24: 1895–903.
Buday A, Orsy P, Godo M, Mozes M, Kokeny G, Lacza Z, et al. Elevated systemic TGF-beta impairs aortic vasomotor function through activation of NADPH oxidase-driven superoxide production and leads to hypertension, myocardial remodeling, and increased plaque formation in apoE(−/−) mice. Am J Physiol Heart Circ Physiol 2010; 299: H386–95.
Yang DH, Ye ZY, Jin B, He XJ, Zhang Q, Zhou WM, et al. Salvianolate inhibits cytokine gene expression in small intestine of cirrhotic rats. World J Gastroenterol 2011; 17: 1903–9.
Wu WY, Wang YP . Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol Sin 2012; 33: 1119–30.
Wang MW, Zhang DF, Tang JJ, Chen B, Wang LS, Yang ZJ, et al. Effects of salvianolate on cardiomyocytic apoptosis and heart function in a swine model of acute myocardial infarction. Zhong Xi Yi Jie He Xue Bao 2009; 7: 140–4.
Nabeebaccus A, Zhang M, Shah AM . NADPH oxidases and cardiac remodelling. Heart Fail Rev 2011; 16: 5–12.
Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, et al. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 2007; 75: 770–81.
Seddon M, Looi YH, Shah AM . Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007; 93: 903–7.
Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG . The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail 2006; 12: 491–8.
Pociask DA, Sime PJ, Brody AR . Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest 2004; 84: 1013–23.
Jiao B, Wang YS, Cheng YN, Gao JJ, Zhang QZ . Valsartan attenuated oxidative stress, decreased MCP-1 and TGF-beta1 expression in glomerular mesangial and epithelial cells induced by high-glucose levels. Biosci Trends 2011; 5: 173–81.
Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, et al. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int 2001; 59: 87–95.
Sui XB, Zhang Q, Qiu HS, Zhou JC, Gu XD, Lu ZX, et al. Mechanism of salvianolate in preventing postoperative intestinal adhesion in rats. Zhong Xi Yi Jie He Xue Bao 2007; 5: 521–5.
Xu J, Fan WH . Effect of salvianolate on migration of human vascular endothelial cells. Zhong Xi Yi Jie He Xue Bao 2003; 1: 211–4.
Xu M, Wang YP, Luo WB, Xuan LJ . Salvianolate inhibits proliferation and endothelin release in cultured rat mesangial cells. Acta Pharmacol Sin 2001; 22: 629–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, Ah., Cao, Q., Chen, Sy. et al. Salvianolate inhibits reactive oxygen species production in H2O2-treated mouse cardiomyocytes in vitro via the TGFβ pathway. Acta Pharmacol Sin 34, 496–500 (2013). https://doi.org/10.1038/aps.2012.209
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.209
Keywords
This article is cited by
-
Effects of salvianolate on microcirculatory disturbance in patients with stable coronary heart disease: study protocol for a randomized controlled trial
Trials (2021)
-
Protective Effects of Salvianolate on Myocardial Injury or Myocardial Infarction after Elective Percutaneous Coronary Intervention in NSTE-ACS Patients: A Randomized Placebo-Controlled Trial
Chinese Journal of Integrative Medicine (2020)
-
A Prospective Randomized Multicenter Controlled Trial on Salvianolate for Treatment of Unstable Angina Pectoris in A Chinese Elderly Population
Chinese Journal of Integrative Medicine (2019)
-
Cost-consequence analysis of salvianolate injection for the treatment of coronary heart disease
Chinese Medicine (2018)
-
Salvianolate reduces murine myocardial ischemia and reperfusion injury via ERK1/2 signaling pathways in vivo
Chinese Journal of Integrative Medicine (2017)