Abstract
Aim:
To investigate the analgesic effect of cobratoxin (CTX), a long-chain α-neurotoxin from Thailand cobra venom, in a rat model of formalin-induced inflammatory pain.
Methods:
Inflammatory pain was induced in SD rats via injecting 5% formalin (50 μL) into the plantar surface of their right hind paw. CTX and other agents were ip administered before formalin injection. The time that the animals spent for licking the injected paw was counted every 5 min for 1 h.
Results:
CTX (25, 34, and 45 μg/kg) exhibited a dose-dependent analgesic effect during the phase 1 (0–15 min) and phase 2 (20–60 min) response induced by formalin. Pretreatment with naloxone (0.5 or 2.5 mg/kg) did not block the analgesic effect of CTX. Pretreatment with atropine at 5 mg/kg, but not at 2.5 mg/kg, antagonized the analgesic effect of CTX. Treatment with the nonselective nAChR antagonist mecamylamine (3 mg/kg) inhibited the analgesic effects of CTX in Phase 1 and Phase 2 responses, while with the selective α7-nAChR antagonist methyllycaconitine (3 mg/kg) antagonized the effect of CTX only in the Phase 1 response. Treatment with the α7-nAChR agonist PNU282987 (3 mg/kg) significantly reduced the formalin-induced phase 2 pain response, but only slightly reduced the Phase 1 pain response.
Conclusion:
The results suggest that CTX exerts an antinociceptive effect in formalin-induced inflammatory pain, which appears to be mediated by mAChR and α7-nAChR.
Similar content being viewed by others
Introduction
Snake venoms and several neurotoxins isolated from venoms have demonstrated potent analgesic activity in animal models of pain. A previous study reported that crotalus dirissus terrificus venom administered subcutaneously inhibited the migration of polymorphonuclear cells to the peritoneal cavity before and after plantar side injection of carrageenan into the mouse right hind paw1, 2. Cobrotoxin3, a short-chain postsynaptic α-neurotoxin isolated from Naja naja atra, is reported to have analgesic activity and is commercially available in China for this purpose4. Cobratoxin (CTX), a neurotoxin isolated from Naja naja kaouthia, is a high-affinity ligand for the alpha 7 nicotinic receptor subtype (α7-nAchR)5, 6, which can conduct Ca2+ ions and thereby directly impact neurotransmitter release7.
Our previous studies found that CTX exhibited a dose-dependent analgesic action in mice as determined by hot-plate and acetic acid writhing tests. The peak effect of analgesia was seen 3 h after CTX administration. Furthermore, naloxone failed to block the analgesic effects of CTX, but atropine did antagonize the analgesia mediated by CTX in the mouse acetic acid writhing test, indicating that the cholinergic, but not opioid system, appears to be involved in the antinociceptive action of CTX8. It is not currently known whether CTX inhibits inflammatory pain. The present study evaluated analgesic effects of CTX in a rat model of inflammatory pain induced by formalin.
Injection of formalin into rat paws is a valid and reliable model of inflammation-mediated nociception. Intradermal injection of formalin into the paw induces a biphasic nociceptive response evidenced by flinching, licking or biting of the affected paw. Two phases of the response can be observed: an early phase starting immediately after injection and lasting for 0–15 min and a late phase from 20 to 60 min after injection. It is now known that the first phase is due to the direct action of formalin on nociceptors, whereas the second phase is mediated by a combination of peripheral input and spinal cord sensitization9, 10, 11. This makes the formalin test a well-accepted animal model for studying pain9. The formalin test is a chemically induced tonic pain model in which the biphasic changes of nociception are considered a molecular basis for neuropathic pain, particularly during the second phase of the test, during which most clinically used drugs against neuropathic pain are active. Opioid analgesics such as codeine and nalbuphine appear to be antinociceptive for both phases12, 13. In contrast, NSAIDs such as diclofenac and lumiracoxib suppress pain only in the second phase14, 15, 16. The present study examined the effects of CTX from Naja naja kaouthia on the nociceptive response by intradermal administration of formalin and the involvement of the opioid and cholinergic systems in its analgesic effects.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 180 to 220 g were purchased from the Experimental Animal Center of Soochow University and housed in a climatically controlled room (temperature 18–22 °C; humidity 40%–80%; 12 h light/dark cycle with lights on at 7:00 AM) with food and water available ad lib. Animals were acclimated to the housing conditions and handled for 3–4 d before experiments. All experiments were performed between 08:00 AM and 16:00 PM. All experimental procedures were conducted according to the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication No 80–23, revised 1996). The experimental procedures were approved by the Committee on Animal Care and Use of Soochow University.
Drug injections
CTX was obtained from ReceptoPharm Inc (Fortlaudale, Florida, USA) and dissolved in 0.9 % saline. The doses of CTX used were 25, 34, and 45 μg/kg, and these were administered ip at a volume of 2 mL/kg 3 h prior to formalin injection. Naloxone, atropine, mecamylamine, methyllycaconitine and PNU282987 were obtained from Sigma (St Louis, MO, USA), dissolved in 0.9% saline and administered ip at a volume of 2 mL/kg. Some mice received an ip injection of naloxone (0.25 and 5 mg/kg) or atropine (0.25 and 5 mg/kg) 2.5 h prior to formalin injection, and some were injected with mecamylamine (3 mg/kg) or methyllycaconitine (3 mg/kg) 1 h before the formalin injection. PNU282987 (3 mg/kg) was administered 30 min prior to formalin injection. The time intervals used for agonist and antagonist administration were adapted from previous studies8. For control rats, 0.9% saline solution was injected at a volume of 2 mL/kg.
Formalin test
For all experiments, animals were habituated to the formalin test environment by placing them in the test apparatus (Plexiglass chamber 16 cm×15 cm×15 cm) for 2 h prior to the injection of formalin. Subjects were then given an ip injection of either CTX or saline, followed by an sc injection of 5% formalin (volume of 50 μL) into the plantar surface of the right hind paw 3 h later. Immediately after the formalin injection, licking time was recorded in 5-min intervals for 1 h.
During each experiment, the time that the animal spent in licking the injected paw was recorded every 5 min for 1 h, and results are shown as the total time spent on licking in each phase. Phase 1 was defined as the period of time beginning immediately after the formalin injection and lasting for 15 min. Phase 2 was defined as beginning 20 min post-formalin injection and lasting until 1 h post-injection. Behaviors during each phase are presented as the sum of the total seconds spent on licking during that phase.
Statistical analysis
All data were analyzed using a one-way ANOVA. Post hoc comparisons were performed using Student's t-test. P<0.05 was considered statistically significant. Calculations were performed using the SPSS 10.0 statistical package.
Results
Formalin response
Intradermal injection of 5% formalin 50 μL into the right hind paw produced a consistent licking response in rats. A biphasic nociceptive behavior occurred immediately in Phase 1 and then diminished gradually (0–15 min), followed by a quiescent period (16–19 min), and then occurred again in Phase 2 (20–60 min) (Figure 1).
Antinociceptive effects of CTX on formalin-induced inflammatory pain
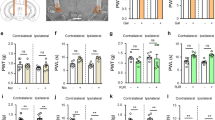
As shown in Figure 1, formalin-evoked biphasic nociceptive responses induced an early, short-lasting response (Phase 1, 0–15 min post-injection) followed by a late, prolonged response (Phase 2, approximately 20–60 min post-injection). Licking time evoked by formalin in both Phase 1 and Phase 2 were reduced in a dose-dependent manner by pretreatment with CTX (20, 34, and 45 μg/kg, ip). Licking time in Phase 1 decreased from 118.60±12.96 s (saline) to 100.40±16.00 s (CTX 20 μg/kg, P>0.05), 86.21±11.14 s (CTX 34 μg/kg, P<0.05), and 65.41±15.09 s (CTX 45 μg/kg, P<0.05). Licking time in Phase 2 decreased from 497.20±62.08 s (saline) to 425.20±35.31 s (CTX 20 μg/kg, P>0.05), 319.41±28.72 s (CTX 34 μg/kg, P<0.05), and 295.01±38.30 s (CTX 45 μg/kg, P<0.05). No side effect was observed in rats after injection of CTX (Figure 2).
Effects of CTX on formalin-induced licking responses. Rats received CTX (25, 34, or 45 μg/kg, ip) or saline vehicle, followed by intradermal injection of formalin 3 h later. Licking time is shown as the mean±SEM from 10 rats per group. Phase 1 was defined as the licking response 0–15 min after formalin, and Phase 2 was established as the licking response 20–60 min after formalin. bP<0.05 compared with the saline group.
Naloxone did not affect the analgesic effects of CTX on formalin-induced pain
Naloxone (0.5 and 2.5 mg/kg, ip) alone had no significant effect on the formalin-induced nociceptive response in either Phase 1 or Phase 2, compared with the saline-treated group. CTX (34 μg/kg, ip) combined with naloxone (0.5 and 2.5 mg/kg, ip) produced significant analgesic effects similar to CTX alone. There was no significant difference between these groups, indicating that naloxone failed to affect the analgesic effects of CTX (Figure 3).
Effects of naloxone on CTX-induced attenuation of the licking response induced by formalin. (A) Effects of systemic naloxone on the formalin-induced pain response. Rats were pretreated with naloxone (0.25, 5 mg/kg, ip) and formalin was injected 2.5 h later. (B) Effects of naloxone on CTX-induced attenuation of the licking response after formalin injection. Thirty minutes after injection of CTX (34 μg/kg, ip), naloxone (0.25, 5 mg/kg, ip) or saline was injected, followed by formalin injection 2.5 h later. Licking time is shown as the mean±SEM from 10 rats per group.
Atropine inhibited the analgesic effects of CTX on formalin-induced pain
As shown in Figure 4, atropine (0.25 and 5 mg/kg) had no significant effect on formalin-induced pain response. When CTX (34 μg/kg) was combined with a small dose of atropine (0.25 mg/kg), licking time in Phases 1 and 2 slightly increased from 94.38±12.99 s to 120.00±10.64 s (Phase 1, P>0.05) and 338.22±34.24 s to 364.25±65.17 s (Phase 2, P>0.05), respectively (Figure 4B). When CTX (34 μg/kg) was combined with a larger dose of atropine (5 mg/kg), licking time in Phases 1 and 2 increased from 94.38±12.99 to 124.40±24.40 s (Phase 1, P<0.05) and 124.40±24.40 s to 460.00±89.20 s (Phase 2, P<0.05) (Figure 4B). These results indicate that a large dose of atropine could antagonize the analgesic and anti-inflammatory effects exerted by CTX.
Effects of atropine on CTX-induced attenuation of the licking response after formalin injection. (A) Effects of systemic atropine on formalin-induced pain response. Rats were pretreated with atropine (0.25 and 5 mg/kg, ip) and formalin was injected 2.5 h later. (B) Effects of atropine on CTX-induced attenuation of licking responses after formalin treatment. Thirty minutes after CTX injection (34 μg/kg, ip), atropine (0.25 and 5 mg/kg, ip) or saline was injected, followed by formalin 2.5 h later. Licking time is shown as the mean±SEM from 10 rats per group. bP<0.05 compared with the CTX (34 μg/kg) alone group.
Mecamylamine inhibited the analgesic effects of CTX on formalin-induced pain
As shown in Figure 5, mecamylamine (3 mg/kg) had a significant effect on the CTX (34 μg/kg, ip)-mediated attenuation of formalin-induced pain response. Licking time in Phase 1 increased from 16.71±3.84 s (CTX 34 μg/kg alone) to 41.20±3.84 s (CTX 34 μg/kg plus mecamylamine 3 mg/kg, P<0.05). Licking time in Phase 2 increased from 94.77±26.09 s (CTX 34 μg/kg alone) to 215.96±35.79 s (CTX 34 μg/kg plus mecamylamine 3 mg/kg, P<0.05, Figure 5). These data suggest that mecamylamine could antagonize the analgesic effects exerted by CTX.
Effects of mecamylamine on CTX-induced attenuation of the licking response after formalin injection. Mecamylamine (3 mg/kg, ip) or saline was administered 2 h after CTX injection (34 μg/kg, ip), followed by formalin 1 h later. Licking time is shown as the mean±SEM from 10 rats per group. bP<0.05, cP<0.01 compared with the saline group; eP<0.05, fP<0.01 compared with the CTX (34 μg/kg) alone group.
Methyllycaconitine inhibited the analgesic effects of CTX on formalin-induced pain in Phase 1
As shown in Figure 6, methyllycaconitine (3 mg/kg) combined with CTX (34 μg/kg, ip) had a significant effect on licking time in Phase 1 compared with CTX (34 μg/kg, ip) alone. Licking time in Phase 1 increased from 16.71±3.84 s (CTX 34 μg/kg) to 47.12±9.92 s (CTX 34 μg/kg and methyllycaconitine 3 mg/kg, P<0.05). However, there was no significant effect on the CTX-mediated reduction in licking time in Phase 2 (Figure 6), indicating that other nAChRs or mAchRs may have participated in the analgesic effects of CTX.
Effects of methyllycaconitine on CTX-induced attenuation of the licking response after formalin injection. Methyllycaconitine (3 mg/kg, ip) or saline was administered 2 h after CTX injection (34 μg/kg, ip), followed by formalin 1 h later. Licking time is shown as the mean±SEM from 10 rats per group. bP<0.05, cP<0.01 compared with the saline group. eP<0.05 compared with the CTX (34 μg/kg) alone group.
PNU282987 inhibited the pain response induced by formalin
PNU282987 had an effect on the formalin-induced nociceptive response (Figure 7). Licking time in Phase 1 slightly decreased from 82.85±11.35 s (saline) to 65.90±16.79 s (PNU282987 3 mg/kg, P>0.05). Licking time in Phase 2 decreased from 295.77±28.39 s (saline) to 186.60±30.49 s (PNU282987 3 mg/kg, P<0.05). These data indicate that CTX may exert its analgesic action against inflammatory pain by activating nicotinic receptors, including α7-nAChR.
Effects of PNU282987 on the formalin-induced licking response. Rats received PNU282987 (3 mg/kg, ip), CTX (34 μg/kg), or saline vehicle, followed by intradermal injection of formalin 1.5 h later. Licking time is shown as the mean±SEM from 10 rats per group. bP<0.05, cP<0.01 compared with the saline group; eP<0.05 compared with the CTX (34 μg/kg) alone group.
Discussion
The mechanism underlying formalin-induced pain behavior involves a complex series of events including peripheral and central biphasic responses. The first phase of the response is driven directly by formalin stimulating to peripheral nociceptors, thereby producing an acute barrage of activity in the dorsal horn. The second phase is thought to be the consequence of ongoing afferent input maintained by inflammatory mediators acting on peripheral nociceptors17, 18, 19 and functional changes in central pain processing20.
In the present study, we evaluated the antinociceptive effects of CTX on formalin-induced inflammatory pain. Our results show that CTX exhibited a dose-dependent analgesic action on formalin-induced biphasic nociceptive behaviors. Naloxone had no impact on CTX-mediated analgesic effects. In contrast, atropine at 5 mg/kg (ip) antagonized the analgesia mediated by CTX. The non-selective nAChR antagonist mecamylamine attenuated the analgesic effects of CTX. These findings indicate that CTX is effective for attenuating nociception induced by inflammation. Chen et al reported that dose-dependent antinociceptive effects of CTX were observed in mice in the acetic acid and hot-plate model, and atropine but not naloxone antagonized the analgesic action of CTX8. The present results are consistent with this study, and together they indicate that the antinociceptive and anti-inflammatory effects of CTX have no association with the opioid system but do involve the cholinergic system.
These data show that atropine antagonized the analgesic and anti-inflammatory effects of CTX on formalin-induced pain in Phase 1 and Phase 2. Atropine is a competitive nonselective antagonist of central and peripheral muscarinic acetylcholine receptors (mAChR). Wang et al have shown that a subtle relationship exists between nicotinic and muscarinic receptors in triggering central cholinergic function21, 22, 23. They also demonstrated that activation of α7 receptors can modulate Muscatine receptors in rat superior cervical ganglion neurons24 and that α-neurotoxins may be considered potent nAChR antagonists, making them efficient paralyzing agents25. Therefore, it is possible that the activation of muscarinic receptors, which leads to antinociceptive effects, may occur after α7 receptors are inhibited by CTX.
It has been proposed that CTX preferentially targets the alpha 7 and alpha 1 nAChRs in nerve and muscle tissue, respectively, and function by preventing the activation of these acetylcholine receptors in pre- and post-synaptic membranes. The involvement of alpha 7 nicotinic receptors in nicotinic analgesia has been assessed in mice. Choline, a α7 receptor agonist, has dose-dependent antinociceptive effects on formalin tests in mice. Methyllycaconitine significantly blocked the effects of choline. These studies suggested that activation of alpha 7 receptors in the central nervous system elicits antinociceptive effects in an acute thermal pain model26. In the present study, we found that mecamylamine blocked CTX-mediated analgesic effects in Phase 1 and Phase 2, while methyllycaconitine inhibited CTX's analgesic action in Phase 1. Moreover, PNU282987 mimicked the effects of CTX in formalin-induced inflammatory pain responses, suggesting that CTX might induce activation of α7-nAChR through indirect mechanisms in vivo. However, methyllycaconitine did not block the formalin-induced Phase 2 nociceptive response. These results indicate that, in addition to α7-nAchR, other mAchRs or nAChRs are also involved in CTX's analgesic action.
In summary, the present study demonstrated that ip injection of CTX, a long-chain α-neurotoxin from Naja naja kaouthia, could dose-dependently decrease formalin-induced inflammatory pain in rats and that this activity is mediated by activation of the cholinergic but not the opioid system.
Author contribution
Zheng-hong QIN and Yan-li LIU designed the research; Yan-li LIU, Gao-na SHI, and Hai-ming LIN performed the research; Paul F REID contributed new analytical tools and reagents; Yan-li LIU, Gao-na SHI, Shi-lin YANG, and Yu-lin FENG analyzed data; Yan-li LIU, Gao-na SHI, Zheng-hong QIN, and Paul F REID wrote the paper.
References
Nunes FP, Sampaio SC, Santoro ML, Sousa-e-Silva MC . Long-lasting anti-inflammatory properties of Crotalus durissus terrificus snake venom in mice. Toxicon 2007; 49: 1090–8.
Farsky SH, Antunes E, Mello SB . Pro and antiinflammatory properties of toxins from animal venoms. Curr drug targets inflamm Allergy 2005; 4: 401–11.
Yang CC . Cobrotoxin: structure and function. J Na Toxins 1999; 8: 221–33.
Grasset E . The cobra neurotoxin, pharmacology and clinical applications in the treatment of pain. Med Hyg (Geneve) 1952; 10: 55–8.
Servent D, Anti-Delbeke S, Gaillard C, Corringer PJ, Changeux JP, Menenz A . Molecular characterization of the specificity of interactions of various neurotoxins on two distinct nicotinic acetylcholine receptors. Eur J Pharmacol 2000; 393: 197–204.
Dajas-Bailador F, Costa G, Dajas F, Emmett. Effects of α-bungarotoxin, α-cobratoxin and fasciculin on the nicotine-evoked release of dopamine in the rat striatum in vivo. Neurochem Intl 1998; 33: 307–12.
Lena C, Changeux JP . Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci 1997; 17: 576–85.
Chen ZX, Zhan HL, Gu ZL, Chen BW, Han R, Reid PF, et al. A long-form α-neurotoxin from cobra venom produces potent opioid independent analgesia1. Acta Pharmacol Sin 2006; 27: 402–8.
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K . The formalin test: an evaluation of the method. Pain 1992; 51: 5–17.
Dubuisson D, Dennis SG . The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977; 4: 161–74.
Wheeler-Aceto H, Cowan A . Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 1991; 104: 35–44.
Ortiz MI, Castro-Olguın J, Pena-Samaniego N, Castaneda-Hernandez G . Probable activation of the opioid receptor-nitric oxide-cyclic GMP-K+ channels pathway by codeine. Pharmacol Biochem Behav 2005; 82: 695–703.
Castaneda-Hernandez G . Possible activation of ATP sensitive K+ channels by nalbuphine on the formalin test. Eur J Pain 2006; 10(supp1): S88.
Malmberg AB, Yaksh TL . Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992; 263: 136–46.
Ortiz MI, Granados-Soto V, Castaneda-Hernandez G . The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol Biochem Behav 2003; 76: 187–95.
Lozano-Cuenca J, Castaneda-Hernandez G, Granados-Soto V . Peripheral and spinal mechanisms of antinociceptive action of lumiracoxib. Eur J Pharmacol 2005; 513: 81–91.
Dickenson AH, Sullivan AF . Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett 1987; 83: 207–11.
Mccall WD, Tanner KD, Levine JD . Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett 1996; 208: 45–8.
Pitcher GM, Henry JL . Second phase of formalin-induced excitation of spinal dorsal horn neurons in spinalized rats is reversed by sciatic nerve block. Eur J Neurosci 2002; 15: 1509–15.
Coderre TJ, Vaccarino AL, Melzack R . Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 1990; 535: 155–8.
Wang H . Modulation by nicotine on muscarinic receptor-effector systems. Acta Pharmacol Sin 1997; 18: 193–7.
Wang H, Cui WY, Liu CG . Regulatory effects of acutely repeated nicotine treatment towards central muscarinic receptors. Life Sci 1996; 59: 1415–21.
Wang H, Cui WY, Liu CH . Modulation by nicotine on binding of cerebral muscarinic receptors with muscarinic agonist and antagonist. Acta Pharmacol Sin 1996; 17: 497–9.
Yin X, Cui W, Hu G, Wang H . Desensitization of alpha7 nicotinic receptors potentiated the inhibitory effect on M-current induced by stimulation of muscarinic receptors in rat superior cervical ganglion neurons. J Neural Transm 2005; 112: 1133–48.
Dutertre S, Lewis RJ . Toxin insights into nicotinic acetylcholine receptors. Biochem Pharmacol 2006; 72: 661–70.
Wang Y, Su DM, Wang RH, Liu Y, Wang H . Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience 2005; 132: 49–56.
Acknowledgements
This work was supported by Pre-research Funding of Soochow University (No Q3132821).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Gn., Liu, Yl., Lin, Hm. et al. Involvement of cholinergic system in suppression of formalin-induced inflammatory pain by cobratoxin. Acta Pharmacol Sin 32, 1233–1238 (2011). https://doi.org/10.1038/aps.2011.65
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.65
Keywords
This article is cited by
-
Cobrotoxin could be an effective therapeutic for COVID-19
Acta Pharmacologica Sinica (2020)