Abstract
Aim:
To study whether genetic polymorphisms of COL1A1 and COL1A2 genes affected the onset of fracture in postmenopausal Chinese women.
Methods:
SNPs in COL1A1 and COL1A2 genes were identified via direct sequencing in 32 unrelated postmenopausal Chinese women. Ten SNPs were genotyped in 1252 postmenopausal Chinese women. The associations were examined using both single-SNP and haplotype tests using logistic regression.
Results:
Twenty four (4 novel) and 28 (7 novel) SNPs were identified in COL1A1 and COL1A2 gene, respectively. The distribution frequencies of 2 SNPs in COL1A1 (rs2075554 and rs2586494) and 3 SNPs in COL1A2 (rs42517, rs1801182, and rs42524) were significantly different from those documented for the European Caucasian population. No significant difference was observed between fracture and control groups with respect to allele frequency or genotype distribution in 9 selected SNPs and haplotype. No significant association was found between fragility fracture and each SNP or haplotype. The results remained the same after additional corrections for other risk factors such as weight, height, and bone mineral density.
Conclusion:
Our results show no association between common genetic variations of COL1A1 and COL1A2 genes and fracture, suggesting the complex genetic background of osteoporotic fractures.
Similar content being viewed by others
Introduction
Osteoporotic fracture is the most serious clinical consequence of osteoporosis. It is a major cause of the public health burden associated with osteoporosis due to the high rates of morbidity and mortality in the elderly and the tremendous health care costs1, 2. The incidence of osteoporotic fracture is increasing rapidly in Asian countries3, 4. The incidence for all osteoporotic fractures and hip fractures was 635/100 000 and 123/100 000 person-years in southern Chinese men, respectively5. Therefore, the elucidation of ways to prevent osteoporotic fracture is an important goal. The most common osteoporotic fracture sites are the hip, wrist and spine6.
Most studies demonstrate a heritability of 50%–80% for bone mineral density (BMD), but fracture heritability has been estimated to be less than 50%7, 8. The risk for osteoporotic fracture is complex and is regulated by both environmental and genetic factors. To date, most genetic studies have focused on surrogate phenotypes of osteoporotic fracture, such as BMD, bone size, bone geometry parameters and bone turnover markers, to search for genes that underlie the susceptibility to osteoporotic fracture9. Although the measurement of BMD has been the best method to identify the risk for osteoporotic fracture, a meta-analysis for the predictive ability of the measurement for hip fracture was reported a false positive rate of 15% and a detection rate of only 50%10. Another study found that the association between genetic variations and wrist fracture was largely independent of BMD8. Many studies have focused on the relationship between candidate genes and BMD, but some studies have found that candidate genes are associated with an increased risk for osteoporotic fracture independently of BMD11, 12, and the genetic correlation between these surrogate phenotypes and osteoporotic fracture is very low8, 13. The underlying genetic factors that cause susceptibility to osteoporotic fracture are generally unknown; therefore, the underlying genetic associations with osteoporotic fracture were assessed in the present study. Due to low fracture heritability7, 13 and difficulty associated with recruiting a large number of individuals with the same fracture type, until now, few studies have performed a direct genetic evaluation of osteoporotic fracture using a case-control method.
Considering the important role of collagen type I in the bone matrix, genes that are related to type I collagen are potential candidates for osteoporotic fracture. Type I collagen acts by binding to two collagen chains, α1 and α2, which are encoded by the COL1A1 and COL1A2 genes, respectively14. There are common polymorphisms in COL1A1: Sp1 (rs1800012) in intron 1, -1997G/T (rs1107946) and -1663in/de1T (rs2412298) have been extensively studied because of their association with the risk for osteoporosis12, 15, 16, 17, 18. Of note, the Sp1 polymorphism is associated with a modest reduction of BMD and a significant increase in the risk for osteoporotic fracture, particularly vertebral fracture12, 16, 18. A recent meta-analysis of clinical studies has confirmed that the Sp1 polymorphism is associated with a higher prevalence of fractures among heterozygote (Ss) and homozygote (ss) in comparison to wild type individuals (SS) and that COL1A1 “s” allele has a stronger association with the risk for fracture than with BMD19. However, the Sp1 polymorphism has not been found in the Chinese population20, 21. The relationship between COL1A2 genotypes and osteoporosis is less well characterized than that between COL1A1 genotypes and osteoporosis. A number of studies have found an association between GT-repeat polymorphisms, Msp I, Pvu II, and EcoR I of the COL1A2 gene and BMD22, 23 or bone size24. However, there are few studies selected osteoporotic fracture as a direct phenotype. Therefore, additional data are required to confirm the potential role of collagen type I genes in the pathogenesis of osteoporotic fracture. In the present study, we aimed to investigate whether the genetic polymorphisms in the COL1A1 and COL1A2 genes affected the onset of osteoporotic fracture independently of clinical risk factors in postmenopausal Chinese women.
Materials and methods
Subjects
The study was approved by the Ethics Committee of the Shanghai Jiao Tong University Affiliated Sixth People's Hospital. All of the subjects included in the study were recruited from a local population in Shanghai City by the Department of Osteoporosis and Bone Diseases, Shanghai Jiao Tong University Affiliated Sixth People's Hospital from 2006 to 2009. All of the subjects signed informed consent documents before participating in the project. After signing, the subjects were assisted with the completion of a structured questionnaire that included information regarding anthropometric variables, lifestyle and medical history. A physical checkup and routine serum examination were performed, and all of the subjects were found to be in good health. Secondary causes of osteoporosis were excluded. Postmenopausal women who had experienced early menopause (before 45 years of age) and those who had undergone ovariectomy or were receiving estrogen replacement therapy or antiosteoporotic drugs were excluded.
Volunteers were recruited from communities in Xuhui district, Shanghai, China and cases of vertebral fractures that were detected by radiographs were screened. Among patients who were seeking medical treatment in the outpatient department, Colles' and hip fractures (including femoral neck fractures and trochanter fractures) were found on the radiographs. The diagnosis was performed by orthopedic surgeons and radiologists according to the information provided by X-rays. In total, 1252 unrelated subjects (627 cases of fragility fracture in the case group and 625 cases in the control group) of postmenopausal Han women between the ages of 45 and 80 years were selected for inclusion in the study. The inclusion criteria for fragility fracture cases were as follows: (i) age <80 years and onset age >45 years; (ii) minimal fractures, usually due to falls from standing height or less; (iii) fracture site location in the lumbar spine, hip and radius; (iv) a diagnosis of osteoporotic fracture identified and confirmed by radiographs that were interpreted by orthopedic surgeons or radiologists and by the Genant semiquantitative method to assess vertebral fractures25. There were 668 fracture times in the fracture group (Table 1): 419 cases were vertebral fractures (composition ratio of 62.7%), 156 cases were Colles' fractures (composition ratio of 22.4%) and 93 cases were hip fractures (composition ratio of 13.9%). All of the subjects with a fracture were considered to have primary osteoporosis. The inclusion criteria for control subjects were an age <80 years, an onset age >45 years and a lack of any fracture history. The exclusion criteria were same as those described in our previously reported study26.
BMD measurements
The BMD (g/cm2) of the left proximal femur (if the individual had a left hip fracture, the right proximal femur was measured) including the total hip, the femoral neck, the trochanter, and Ward's triangle were measured using dual-energy X-ray absorptiometry (DXA) on a Hologic QDR 2000 (Hologic, Bedford, MA, USA) and a Lunar Prodigy GE densitometer (Lunar Corp, Madison, WI, USA). Both of the scanners were calibrated daily, and the coefficient of variability (CV) values for the DXA measurements at L1-4, the total hip, and the femoral neck were 0.9%, 0.8%, and 1.93%, respectively, for the Hologic QDR 200026 and 1.39%, 0.7%, and 2.22%, respectively, for the Lunar Prodigy27. The long-term reproducibility of the DXA data during the trial, which was based on phantom measurements that were repeated weekly, was 0.45%. Height and weight were measured using standardized equipment.
Sequencing analysis of the COL1A1 and COL1A2 genes
Genomic DNA was isolated from peripheral blood leukocytes using conventional methods. Single nucleotide polymorphisms (SNP) screening was conducted by DNA sequencing of the entire gene sequence in 32 unrelated healthy postmenopausal Chinese women using the ABI Prism 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The primers used for the amplification and sequencing analysis were designed based on GenBank sequences (reference sequence of COL1A1 mRNA: NM_000088.3 and COL1A2 mRNA: NM_000089.3). Sequence variants were verified using the Phred/Phrap/Consed package and Polyphred28, 29.
SNP selection and genotyping
According to the sequencing analysis of the COL1A1 and COL1A2 genes and specific criteria, ten SNPs in two genes were selected. SNPs in the two genes were selected based on the following criteria: (i) degree of heterozygosity (minor allele frequency (MAF) >10%); (ii) r2=0.8; (iii) denoted as tagSNPs; (iv) amino acid mutations, missense mutations: rs1800215 (Ala1075Thr) and rs42524 (Pro549Ala), and synonymous mutations: rs1801182 (Thr29Thr) and rs1800248 (Gly1045Gly). Based on these criteria, we selected five SNPs in the COL1A1 gene, including SNP 3 (rs2075554), SNP 7 (rs2586494), SNP 18 (rs1800215), SNP 19 (rs2586488) and SNP 23 (rs2277632), and five SNPs in COL1A2, including SNP 1 (rs1801182), SNP 9 (rs42517), SNP 18 (rs42524), SNP 19 (rs421587), and SNP 27 (rs1800248). Ten SNPs were genotyped using the TaqMan assay, for which the primer and probe sequences were optimized using the SNP Assay-by-Design service provided by Applied Biosystems. The genotype for every sample was named according to the ratio of the fluorescence intensities of the two dyes. The reactions were performed with a Mx3000P Real-Time PCR System (Stratagene, CA, USA) using 20 ng of genomic DNA in a 5-μL reaction volume per well.
LD and haplotype analysis
Haplotypes were constructed from the population genotype data using the algorithm of Stephens with the Phase program version 2.0.230. The linkage disequilibrium block structure was examined using Haploview version 3.231. We examined Lewontin's D' and the LD coefficient r2 between all of the pairs of biallelic loci. The genotype and haplotype frequencies were calculated for 32 unrelated subjects and 1252 postmenopausal women.
Statistical analysis
Statistical analyses were performed using SPSS version 11.0 (SPSS, Chicago, IL, USA). The allele frequencies for each SNP were calculated by counting the alleles, and the Hardy-Weinberg equilibrium was assessed by Chi-squared analysis.
Because of the different DXA devices that were used to measure BMD in the 1252 subjects (some were measured by Hologic QDR 2000 and others by Lunar Prodigy GE), the absolute BMD values could not be compared. Therefore, we transformed the absolute value determined for the femoral neck BMD to a Z-score of the neck BMD using the BMD reference database for a previously reported Shanghai population26, 27.
The age, height, weight, and femoral neck BMD (Z-score) for the nine genotypes in the two groups were analyzed by analysis of variance (ANOVA). Analysis of covariance (ANCOVA) was used to analyze the association of the nine genotypes or haplotypes with femoral neck BMD while simultaneously adjusting the height, weight, and years since menopause (YSM) as covariates. A Chi-squared analysis was used to test the differences among the nine SNP genotype frequencies between the case and control groups. By adjusting for age, height, weight, and YSM and BMD as risk factors, a logistic regression was used to analyze the association of the nine genotypes or haplotypes with the risk for osteoporotic fracture. P<0.05 was considered statistically significant in all of the analyses.
Results
Basic characteristics of the subjects
1252 postmenopausal women were included in the study: 627 osteoporotic fracture cases and 625 controls. The basic characteristics of the study subjects are shown in Table 2. Differences in age, height, weight, YSM and femoral neck BMD (Z-score) were found in the two groups according to a t-test analysis. The average age in the case group [(70.4±9.2) years] was older than that in the control group [(60.6±6.7) years] (P<0.01); the average height in the case group [(151.6±6.9) cm] was less than that in the control group [(155.3±5.6) cm] (P<0.01); the average weight in the case group [(55.7±9.7) kg] was lower than that in the control group [(57.6±8.3) kg] (P<0.01); the average femoral neck BMD was lower in the case group (Z-score: -0.42±1.07) than in the control group (Z-score: -0.08±0.97) (P<0.01); and YSM was longer in the case group [(16.9±7.1) years] than in the control group [(15.1±6.6) years] (P<0.05). No significant differences were observed between the two groups with respect to BMI (P >0.05).
SNP genotyping and linkage disequilibrium
SNPs were genotyped for a sample of 32 unrelated individuals who were randomly selected among 1252 subjects in our population, and 24 and 28 SNPs were identified in the COL1A1 and COL1A2 genes, respectively. Twenty SNPs in the COL1A1 gene were found in dbSNP of the NCBI database, but four SNPs were novel. Twenty-one SNPs in the COL1A2 gene were found in dbSNP of the NCBI database, but seven SNPs were novel. All of the SNPs demonstrated MAF of >1.0% in our population, and the genotype frequencies of these SNPs did not deviate from Hardy-Weinberg equilibrium (P>0.05; Tables 3 and 4). The frequencies of each SNP are shown in Tables 3 and 4. According to these genotype frequencies and the above mentioned criteria, five SNPs (rs2075554, rs2586494, rs1800215, rs2586488, and rs2277632) in the COL1A1 gene and five SNPs (rs1801182, rs42517, rs42524, rs421587, and rs1800248) in the COL1A2 gene were subsequently measured.
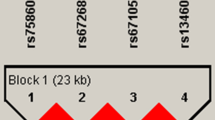
The data for ten SNPs in the COL1A1 and COL1A2 genes are summarized in Table 5. Among those SNPs, rs1800215 in the COL1A1 gene deviated from Hardy-Weinberg equilibrium, as determined by chi-squared analysis (χ2=85.63, P<0.001), and therefore, this SNP was excluded from the subsequent statistical analysis. The genotype frequencies of the other nine SNPs did not deviate significantly from Hardy-Weinberg equilibrium (P>0.05). The LD patterns of COL1A1 and COL1A2 are shown in Figures 1 and 2. In the COL1A1 gene, based on the D' values (D'=0.87), we identified one block with a high LD that ranged from intron 44 to intron 48 and included rs2586488 and rs2277632 (Figure 1A). When the haplotypes were reconstructed with the inclusion of rs2586488 and rs2277632, four potential haplotypes were obtained for the COL1A1 gene (Figure 1B). In the COL1A2 gene, only a single LD block was identified based on the D' values (D'=0.87), and it ranged from exon 3 to exon 47 and included rs1801182, rs42517, rs42524, rs421587, and rs1800248 (Figure 2A). When the haplotypes were reconstructed with the inclusion of these five SNPs in the COL1A2 gene, six possible haplotypes were obtained (Figure 2B).
LD patterns for the COL1A1 gene in 1252 subjects. Squares in red indicate a strong LD. Values in the cells represent the pairwise degrees of LD indicated by D'×100 when D'<1. (A) 1 to 4 represent rs2075554, rs2586494, rs2586488, and rs2277632, respectively. (B) Haplotype frequencies for the COL1A1 gene.
LD patterns for the COL1A2 gene in 1252 subjects. Squares in red indicate a strong LD. Values in the cells represent the pairwise degrees of LD indicated by D'×100 when D'<1. (A) 1 to 5 represents rs1801182, rs42517, rs42524, rs421587, and rs1800248, respectively. (B) Haplotype frequencies for the COL1A2 gene.
Association between SNP and haplotype and BMD in postmenopausal women
There were no differences in the femoral neck BMD values (Z-score) determined for the 9 SNP genotypes according to an ANOVA analysis (data not shown). The results for the association between the COL1A1 and COL1A2 genotypes and BMD were determined by ANCOVA. Following an adjustment for height, weight and YSM as covariates, no significant results were obtained for the association between each SNP and the femoral neck BMD (data not shown). Using ANCOVA to analyze the association of the haplotypes with femoral neck BMD, we failed to detect any differences in femoral neck BMD among the various haplotypes (data not shown).
SNP and haplotype analyses for osteoporotic fractures
Using Chi-squared analysis, no differences were observed between the case and control groups with respect to the nine SNP genotype frequencies (Table 6). The same method was used to evaluate differences between the case and control groups with respect to the nine SNP allele frequencies, but no differences were detected (data not shown). Following an adjustment for age, height, weight, YSM, and BMD as risk factors, we failed to find any significant associations between osteoporotic fracture and each SNP according to a logistic regression (data not shown). We further investigated the relationship between various haplotypes and osteoporotic fracture using logistic regression. No significant association was found between any of the haplotypes and osteoporotic fracture (Table 7). However, height, weight and femoral neck BMD (Z-score) were found to be risk factors for osteoporotic fracture (Table 7). Meanwhile, we investigated the relationship between various haplotypes and vertebral, wrist and hip fracture using logistic regression, respectively. Also no significant association was found between any of the haplotypes and various sites fracture (data not shown).
To eliminate the age differences that might be produced by false-negative results, we matched 627 cases in the fracture group to 625 cases in the control group. After matching the two groups according to age, 813 postmenopausal women among the 1252 cases were included (402 cases of fragility fracture in the case group and 411 cases in the control group). And we used the same method as the above statistical analyses to analyze association between polymorphisms and haplotypes in COL1A1 and COL1A2 genes and osteoporotic fracture in 813 postmenopausal women. No differences were found in neck BMD among the various SNPs or any haplotypes (data not shown). No significant association was found between polymorphisms or any of the haplotypes and osteoporotic fracture (data not shown).
Discussion
This study is the first to report the distribution by DNA sequencing of the entire COL1A1 and COL1A2 genes in healthy postmenopausal Chinese women. In the present study, we found four novel SNPs in the COL1A1 gene and seven novel SNPs in the COL1A2 gene and that the distribution frequencies of two SNPs in COL1A1 (rs2075554 and rs2586494) and three SNPs in COL1A2 (rs42517, rs1801182, and rs42524) were significantly different from those documented for the European Caucasian population shown in the HapMap database. This result suggests that the allele distribution of the two gene frequencies differs among various ethnicities.
The extent to which genetic information can improve the prognosis of a fracture is largely unknown. Based on the importance of collagen type I in the bone matrix, we explored the associations of the COL1A1 and COL1A2 genes with osteoporotic fracture in postmenopausal Chinese women.
The COL1A1 gene has been widely studied in previous reports, but inconsistent results have been obtained, even for the same allele. For example, some studies have found that the Sp1 polymorphism in the COL1A1 gene contributes to susceptibility to vertebral fracture or BMD12, 15, 16, 18, but other studies have reported that there is no such association20, 21. These discordant findings may be due to the inclusion of study subjects from populations with different ethnicities. Due to the lack of the Sp1 polymorphism in Chinese women20, 21, this SNP was not evaluated in the present study. Few studies have investigated the association among other SNPs (excluding the Sp1 polymorphism) in COL1A1 and osteoporotic fracture or BMD16, 17. Gareia-Giralt et al16 found two novel SNPs, -1997G/T (rs1107946), and -1663in/de1T (rs2412298), in COL1A1 that were located in the upstream regulatory region of the gene in postmenopausal Spanish women; the authors suggested that -1997G/T had a significant association with BMD. Jin et al17 found that the -1997G/T, -1663in/delT, and +1245G/T polymorphisms affected BMD and that the binding of several regulatory factors (Nmp4 and Osterix) regulated the transcription of COL1A1. In the present study, we evaluated four SNPs in the COL1A1 gene (rs2075554, rs2586494, rs2586488, and rs2277632) and failed to find an association between single-SNP or haplotype analysis and osteoporotic fracture.
Until now, most studies have investigated the association between SNPs in COL1A2 and BMD or other surrogate phenotypes22, 23, 24, 32. To our knowledge, this is the first study to investigate the association of COL1A2 with osteoporotic fracture. Our results revealed that there was no individual SNP or any of haplotypes in COL1A2 associated with osteoporotic fracture. Lindahl et al23 discovered interactions between the COL1A2 gene and BMD in older men from Sweden, the United Kingdom and Hong Kong (n=2004). Their results demonstrated that rs42524 was significantly associated with BMD and older men with a CC or GG genotype had a higher BMD than men who carried the CG genotype. In the present study, the same SNP was evaluated and failed to demonstrate any association with BMD or the risk for osteoporotic fracture in postmenopausal women. These inconsistent findings may be explained by the use of study subjects with different ethnicities and genders. Lau et al22 found that Pvu II and EcoR I in the COL1A2 gene was associated with BMD in elderly men in Hong Kong, but they did not find any association between these two SNPs and BMD in 450 postmenopausal women. Lei et al24 analyzed Msp I in the COL1A2 gene and osteoporosis according to another phenotype (bone size). Using the quantitative transmission disequilibrium test (QTDT) in Chinese population, they found that this SNP was associated with femoral neck bone size. These studies, which were performed in Chinese population, used BMD or bone size as the study phenotype, in contrast to the use of osteoporotic fracture as the phenotype in the present study. Therefore, our results cannot be compared with the above findings obtained for the Chinese population.
A shortcoming of association studies is the recognition of population admixtures that yield false-positive or false-negative genetic effects for complex traits, which can have significant adverse effects on the results33. To eliminate the age stratification that might be produced by false-negative or false-positive results, we matched 402 cases in the fracture group to 411 cases in the control group. But we also did not find differences in neck BMD among the various SNPs or any haplotypes, and also failed to find significant association between polymorphisms or any of the haplotypes and osteoporotic fracture.
Although osteoporotic fracture is a heritable trait, many studies have also indicated that fracture is partly governed by genetic factors that are distinct from bone mineralization as measured by the BMD8, 10, 11, 13. Tran et al34 also found that genetic profiling could enhance the predictive accuracy of fracture prognosis by comparison of clinical risk factors and 50 genes in 858 men and 1358 women with fragility fractures. Because fractures typically occur later in life, it is difficult to study the genetics of the osteoporotic fracture phenotype11, 13, 35. Therefore, quantitative risk factors such as BMD, bone geometry parameters and bone turnover markers are used to predict the risk for osteoporotic fractures9, 10. However, it is important to note that none of these surrogate phenotypes are ideal for a genetic study36, because these phenotypes could not entirely replace osteoporotic fractures. Furthermore, recent genome-wide association study (GWAS) showed that rs4355801 near the TNFRSF11B (osteoprotegerin) gene and rs3736228 in the LRP5 gene were associated with BMD, only rs3736228 was associated with the risk of osteoporotic fractures identified in 314 075 SNPs in 6463 people37. This study also did not find associations between SNPs in COL1A1 and COL1A2 genes and osteoporotic fracture or BMD37, and was accordant with our result.
One of the strengths of the present study was that the fracture data obtained for the case group was verified by hospital radiographs rather than comprising a collection of self-reported fractures. Another strength was that the SNPs in our study were gotten in the sequencing analysis of COL1A1 and COL1A2 genes in postmenopausal Chinese women. Our study has several limitations. First, because we selected the most informative SNPs, which demonstrated a high MAF and r2, some causal variants (such as rare SNPs) might have been overlooked. Second, a number of other risk factors were not taken into account, such as the fall frequency.
In conclusion, we tested the association between SNPs and haplotypes in COL1A1 and COL1A2 genes with osteoporotic fracture in postmenopausal Chinese women. Our results failed to find the association between each of the 9 SNPs and haplotypes in COL1A1 and COL1A2 genes and osteoporotic fracture. Although our findings must be confirmed in other populations, the present results suggest that the common genetic variants in COL1A1 and COL1A2 are not major contributors to the risk for osteoporotic fracture in postmenopausal Chinese women. To confirm whether the SNPs in COL1A1 and COL1A2 can be used as a genetic means of determining the risk for osteoporotic fracture requires a large number of sample cases from populations that comprise different ethnicities and ages. In addition, lower and rare SNPs information in these two genes needs further research.
Author contribution
Wei-wei HU measured all SNPs, carried out all statistical analyses and drafted the manuscript. Zhen-lin ZHANG conceived and designed the study and revised the manuscript. Jin-wei HE guided the work of the genetics laboratory and guaranteed and confirmed the quality of the genetic data. Chun WANG, Hao ZHANG, and Hua YUE guided the work of the genetics laboratory. Jie-mei GU, Wen-zhen FU, Yun-qiu HU, Miao LI, and Yu-juan LIU carried out the field work including sample and data collection. Yao-hua KE coordinated the DNA sample collection. All authors read and approved the final manuscript.
References
Cummings SR, Melton LJ . Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359: 1761–7.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A . Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22: 465–75.
Xu L, Cummings SR, Qin MW, Zhao XH, Chen XS, Michael N, et al. Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res 2000; 15: 2019–25.
Lau EM, Lee JK, Suriwongpaisal P, Saw SM, Das De S, Khir A, et al. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS). Osteoporos Int 2001; 12: 239–43.
Bow CH, Tsang SW, Loong CH, Soong CS, Yeung SC, Kung AW . Bone mineral density enhances use of clinical risk factors in predicting ten-year risk of osteoporotic fractures in Chinese men: the Hong Kong Osteoporosis Study. Osteoporos Int DOI: 10.1007/s00198-010-1490-0.
Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993; 94: 646–50.
Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL . Genetic liability to fractures in the elderly. Arch Intern Med 2005; 165: 1825–30.
Deng HW, Chen WM, Recker S, Stegman MR, Li JL, Davies KM, et al. Genetic determination of Colles' fracture and differential bone mass in women with and without Colles' fracture. J Bone Miner Res 2000; 15: 1243–52.
Wang JT, Guo Y, Yang TL, Xu XH, Dong SS, Li M, et al. Polymorphisms in the estrogen receptor genes are associated with hip fractures in Chinese. Bone 2008; 43: 910–4.
Chen Y, Shen H, Yang F, Liu PY, Tang N, Recker RR, et al. Choice of study phenotype in osteoporosis genetic research. J Bone Miner Metab 2009; 27: 121–6.
Garnero P, Munoz F, Borel O, Sornay-Rendu E, Delmas PD . Vitamin D receptor gene polymorphisms are associated with the risk of fractures in postmenopausal women, independently of bone mineral density. J Clin Endocrinol Metab 2005; 90: 4829–35.
Nguyen TV, Esteban LM, White CP, Grant SF, Center JR, Gardiner EM, et al. Contribution of the collagen I alpha1 and vitamin D receptor genes to the risk of hip fracture in elderly women. J Clin Endocrinol Metab 2005; 90: 6575–9.
Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, et al. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol 2002; 22: 12–25.
Retief E, Parker MI, Retief AE . Regional chromosome mapping of human collagen genes alpha 2(I) and alpha 1(I) (COLIA2 and COLIA1). Hum Genet 1985; 69: 304–8.
Tran BN, Nguyen ND, Center JR, Eisman JA, Nguyen TV . Enhancement of absolute fracture risk prognosis with genetic marker: the collagen I alpha 1 gene. Calcif Tissue Int 2009; 85: 379–88.
Garcia-Giralt N, Nogues X, Enjuanes A, Puig J, Mellibovsky L, Bay-Jensen A, et al. Two new single-nucleotide polymorphisms in the COL1A1 upstream regulatory region and their relationship to bone mineral density. J Bone Miner Res 2002; 17: 384–93.
Jin H, van't Hof RJ, Albagha OM, Ralston SH . Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet 2009; 18: 2729–38.
Jin H, Evangelou E, Ioannidis JP, Ralston SH . Polymorphisms in the 5′ flank of COL1A1 gene and osteoporosis: meta-analysis of published studies. Osteoporos Int 2011; 22: 911–21.
Mann V, Ralston SH . Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone 2003; 32: 711–7.
Lambrinoudaki I, Kung AW . Absence of high-risk “s” allele associated with osteoporosis at the intronic SP1 binding-site of collagen I alpha1 gene in Southern Chinese. J Endocrinol Invest 2001; 24: 499–502.
Lei SF, Deng FY, Liu XH, Huang QR, Qin Y, Zhou Q, et al. Polymorphisms of four bone mineral density candidate genes in Chinese populations and comparison with other populations of different ethnicity. J Bone Miner Metab 2003; 21: 34–42.
Lau EM, Choy DT, Li M, Woo J, Chung T, Sham A . The relationship between COL1A1 polymorphisms (Sp1) and COL1A2 polymorphisms (Eco R1 and Puv II) with bone mineral density in Chinese men and women. Calcif Tissue Int 2004; 75: 133–7.
Lindahl K, Rubin CJ, Brandstrom H, Karlsson MK, Holmberg A, Ohlsson C, et al. Heterozygosity for a coding SNP in COL1A2 confers a lower BMD and an increased stroke risk. Biochem Biophys Res Commun 2009; 384: 501–5.
Lei SF, Deng FY, Xiao SM, Chen XD, Deng HW . Association and haplotype analyses of the COL1A2 and ER-alpha gene polymorphisms with bone size and height in Chinese. Bone 2005; 36: 533–41.
Genant HK, Wu CY, van Kuijk C, Nevitt MC . Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993; 8: 1137–48.
Zhang ZL, He JW, Qin YJ, Hu YQ, Li M, Zhang H, et al. Association between myostatin gene polymorphisms and peak BMD variation in Chinese nuclear families. Osteoporos Int 2008; 19: 39–47.
Gao G, Zhang ZL, Zhang H, Hu WW, Huang QR, Lu JH, et al. Hip axis length changes in 10,554 males and females and the association with femoral neck fracture. J Clin Densitom 2008; 11: 360–6.
Gordon D, Abajian C, Green P . Consed: a graphical tool for sequence finishing. Genome Res 1998; 8: 195–202.
Ewing B, Hillier L, Wendl MC, Green P . Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 1998; 8: 175–85.
Stephens M, Smith NJ, Donnelly P . A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001; 68: 978–89.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–5.
Lei SF, Deng FY, Dvornyk V, Liu MY, Xiao SM, Jiang DK, et al. The (GT)n polymorphism and haplotype of the COL1A2 gene, but not the (AAAG)n polymorphism of the PTHR1 gene, are associated with bone mineral density in Chinese. Hum Genet 2005; 116: 200–7.
Deng HW . Population admixture may appear to mask, change or reverse genetic effects of genes underlying complex traits. Genetics 2001; 159: 1319–23.
Tran BN, Nguyen ND, Nguyen VX, Center JR, Eisman JA, Nguyen TV . Genetic profiling and individualized prognosis of fracture. J Bone Miner Res 2011; 26: 414–9.
Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD . Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res 2005; 20: 67–74.
Karasik D, Hsu YH, Zhou Y, Cupples LA, Kiel DP, Demissie S . Genome-wide pleiotropy of osteoporosis-related phenotypes: the framingham study. J Bone Miner Res 2010; 25: 1555–63.
Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 2008; 371: 1505–12.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (No 30570891, 30771019, 30800387, 81070692, and 81000360), the Program of Shanghai awarded to the chief scientist (No 08XD1403000) and STCSM10DZ1950100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Ww., He, Jw., Zhang, H. et al. No association between polymorphisms and haplotypes of COL1A1 and COL1A2 genes and osteoporotic fracture in postmenopausal Chinese women. Acta Pharmacol Sin 32, 947–955 (2011). https://doi.org/10.1038/aps.2011.37
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.37
Keywords
This article is cited by
-
Sp1 Binding Site Polymorphism at COL1A1 Gene and Its Relation to Bone Mineral Density for Osteoporosis Risk Factor Among the Sikkimese Men and Women of Northeast India
Indian Journal of Clinical Biochemistry (2019)
-
Association between vitamin D receptor gene polymorphisms (Fok1 and Bsm1) and osteoporosis: a systematic review
Journal of Diabetes & Metabolic Disorders (2014)