Abstract
Aim:
To develop a rational immunotherapy against tumor metastasis by combining a Toll-like-receptor 2 (TLR2)-neutralizing antibody with a TLR9 agonist CpG ODN, and then investigate the mechanism of action for this combinational regimen.
Methods:
After mouse melanoma B16-F10 cell inoculation, female C57BL/6 mice were treated with either CpG ODN (0.5 mg/kg) or the anti-TLR2 antibody (200 μg/kg), or with a combination of the two agents. Pulmonary metastases were evaluated by counting metastatic nodes on the lung surface using anatomical microscopy. Flow cytometry was used to evaluate the cytotoxicity of the immune cells in tumor-draining lymph nodes, the cell population in the spleen, and the infiltration of immune cells within the lungs. Cytokine and enzyme expression in the lung tissue was evaluated using ELISA or immunostaining.
Results:
Anti-metastatic effects were detected in mice treated with either CpG ODN or the anti-TLR2 antibody alone. However, treatment with CpG ODN plus the anti-TLR2 antibody synergistically suppressed the metastasis as compared with treatment with either single agent. The combinational treatment resulted in enhanced infiltration of natural killer cells and cytotoxic T cells, reduced recruitment of type 2 macrophages and Tregs, and decreased expression of immunosuppressive factors including TGF-β1, cyclooxygenase-2 and indoleamine 2,3-dioxygenase, thus stimulated tumor cytotoxicity and suppressed metastasis. The anti-metastatic effect of the combinational regimen was further confirmed in spontaneous metastatic mouse model of Lewis lung carcinoma.
Conclusion:
Our studies suggest that combining a TLR9 agonist with an anti-TLR2 antibody, which eliminates immunosuppressive factors from the tumor environment, is critical for an effective anti-metastatic immunotherapy.
Similar content being viewed by others
Introduction
During the last two decades, significant advances have been made in the field of cancer immunotherapy, and numerous strategies have been developed to provide a tumor-specific immune response1. However, the success of these strategies in clinical trials remains limited. Increasing evidence has shown that not only are there insufficient numbers of anti-tumor effector cells but that there are also too many immunosuppressive factors in tumor environment, which culminates in the failure to eradicate tumor cells2, 3. Thus, a rational anti-cancer immunotherapeutic strategy should include both anti-tumor immune elements and elements able to eliminate tumor-induced immunosuppressive factors4.
Toll-like receptors (TLRs) recognize pathogen- or damage-associated molecular patterns (PAMPs or DAMPs) to initiate innate and adaptive immune responses against pathogens and cancer cells5. In addition to their importance in pathogen and transformed cell elimination, TLRs are of great importance in mediating chronic inflammation and immune tolerance6. TLR targeting has been promising as an effective regimen for disease treatment. The TLR9 agonist CpG ODN was shown to have encouraging anti-tumor activity mediated by simulation of anti-tumor immunity in a number of experimental models. TLR9 agonists induce the activation of plasmacytoid DCs and B cells and stimulate potent Th1-type immune responses. Therefore, TLR9 agonists have been used or are currently under development as monotherapies or in combination with other anti-cancer therapies7. However, there have been disappointing results from phase III trials combining TLR9 agonists with chemotherapeutic agents for treatment of non-small-cell lung cancer, with little evidence of tumor regression or remission compared with the control7, 8. A recent study has suggested that immune suppression induced by tumor cells inhibits the activation of the immune system by TLR9 agonists9.
Recent data indicate that many endogenous molecules released by transformed or damaged cells act as alarm signals to stimulate TLRs and induce chronic inflammation to facilitate tumor growth and metastasis10. TLR2 has been identified as one of the important mediators inducing the immunosuppressive response11. Studies by Kim et al and our own group indicate that blocking TLR2 activity is a novel therapeutic strategy for anti-metastasis that combats the immunosuppressive microenvironment12, 13. These findings collectively suggest that a combination of a TLR2-neutralizing antibody with a TLR9 agonist CpG ODN may produce greater anti-metastatic activity than either treatment alone. In this study, we demonstrate that a TLR9 agonist CpG ODN, which can initiate anti-tumor immunity, combined with a TLR2-neutralizing antibody, which can eliminate inhibitory immune factors from tumor tissue, synergistically act to induce an intense anti-metastatic effect compared with either agent alone. Our studies suggest that combining an immune stimulatory agent with an agent that eliminates immunosuppressive factors from the tumor environment is a rational strategy for designing an effective immunotherapeutic regimen against tumor metastasis.
Materials and methods
Reagents
CpG ODN 1826 (5′-tcc atg acg ttc ctg acg tt-3′, phosphorothioate) and the CpG ODN 1826 control (5′-tcc atg agc ttc ctg agc tt-3′, phosphorothioate) were synthesized by Beijing SBS Corporation. FITC-, PE-, or PE-cy5-conjugated anti-CD3, CD4, CD8, CD25, Foxp3, F4/80, CD206, NK1.1, interferon (IFN)-γ, IL-4, IgG2b, and IgG2a mAb were purchased from eBioscience (San Diego, CA, USA). Anti-Indoleamine 2,3-Dioxygenase (IDO) and Cyclooxygenase-2 (COX2) antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). The neutralizing TLR2 mAb was from R&D System Inc (Minneapolis, MN, USA).
Cell culture
The mouse melanoma cell line B16F10 and the Lewis lung carcinoma cells were cultured in RPMI-1640 (Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 2 g/L Na2CO3, 100 units/mL penicillin, 50 μg/mL gentamicin, and 10% FBS at 37 °C in 5% CO2. These two cells were kindly donated by Prof Rui HAN of the Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Preparation of animal models
Female C57BL/6 mice were purchased from Vital River Lab Animal Technology, Co Ltd (Beijing, China) and maintained under standard conditions in an animal facility at the Institute of Materia Medica. Animal care and experimentation were conducted in accordance with the guidelines of the Institutional Committee for the Ethics of Animal Care and Treatment in Biomedical Research of the Chinese Academy of Medical Sciences and Peking Union Medical College. All mice used in these studies weighed between 16 and 18 g.
To generate a mouse model of pulmonary metastasis, B16F10 cells were trypsinized and resuspended in a PBS solution at a density of 6.25×105 cells/mL. Then, 200 μL of the suspension was injected into the lateral tail vein of each mouse. The mice were euthanized with an overdose of anesthesia on the 21st day after inoculation, and a whole lung was extracted for calibrating the lung index by lung weight (mg) per body weight (g). An anatomical microscopic metastasis quantization was performed by counting the metastatic nodes on the surface of the whole lung. The lungs were then fixed with 4% paraformaldehyde to prepare for histological analysis.
B16F10 melanoma cells were inoculated on day 0. The TLR2-neutralizing (200 μg/kg), anti-IgG antibody (200 μg/kg), CpG (0.5 mg/kg) and CpG control (0.5 mg/kg) were injected on day 3. The treatment with CpG or CpG control was repeated every three days and the treatment with TLR2-neutralizing or anti-IgG antibody was repeated every seven days12, 14. The sham and control mice received a PBS vehicle treatment alone.
To determine the impact of the TLR2-neutralizing antibody plus CpG treatment on tumor cell growth and spontaneous metastasis, 2×106 Lewis lung carcinoma cells were resuspended in 100 μL PBS and subcutaneously injected into the right flank of mice to establish tumors. Tumor volume was calculated as (length×width)2/2 every four days. When small lumps ranging in diameter from 1 to 2 mm were palpated on the 7th day, the mice were randomly divided into four equally sized groups. The dosage and frequency of the TLR2-neutralizing antibody, IgG, CpG, and CpG control were the same as the B16 experiment. Mice treated with cyclophosphamide (40 mg/kg, ip) every two days were used as a positive control. At 24 days post-tumor inoculation, the mice were sacrificed by excessive anesthesia. The primary tumors and the lungs were surgically removed and weighed. The lung metastatic nodes were counted using anatomical microscopy.
Immunohistochemistry
The left lower lobe of the lung was isolated, fixed, paraffin embedded and coronally sliced into 4-μm sections. The protocols for immunohistochemical staining for IDO and COX2 have been described previously12 (the dilution was 1:250 for primary and secondary antibodies). The protein signal intensity was determined by Image-Pro Plus image analysis software. The reported final integrated optical densities (IOD) represent averages from 10 non-overlapping images of each lung specimen.
Cytotoxicity assay
Tumor draining lymph nodes (TDLNs) from B16 mice treated with the indicated agents were isolated and tested for cytotoxicity against B16F10 tumor cells using a standard flow cytometry assay15. To determine the cytotoxicity of immune cells from TDLNs, B16F10 melanoma cells were labeled with 5 μmol/L of CFSE [5- or 6-(N-Succinimidyloxycarbonyl)-3′,6′-O,O′-diacetylfluorescein, Dojindo Mol Technol, Inc] and cultured with the TDLNs at 3 different effector to target (E:T) ratios (50:1, 25:1, and 12.5:1) in triplicate wells for 4 h. The cells were harvested and stained with propidium iodide (PI). Dead target cells were detected as PI+CFSE+ cells using flow cytometry. Cytotoxicity, defined as the percent cytotoxicity in each sample, was calculated based on each E:T ratio. The dose-response curves of percent-specific lysis versus E:T ratios for each TDLN sample were evaluated.
Intracellular cytokine staining
Spleens were collected from the mice treated with the previously described agents. The spleens were minced over a 70-μm cell strainer, and the red blood cells were lysed using lysis buffer (Biolegend, San Diego). The splenocytes were stimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (1 mmol/L) for 6 h in the presence of monensin (3.4 ng/mL) prior to staining16. The cells were stained with FITC-, PE-, and/or PE-cy5-conjugated mAb against CD4, IFN-γ and IL-4 (Dilution was 1:50) (Th1 cells: CD4+IFN-γ+ cells; Th2 cells: CD4+IL-4+ cells). Samples were determined by using a FACS Canto analyzer, and data were analyzed using CellQuest software (Becton Dickinson, San Joes, USA).
Flow cytometry
The lungs were perfused through the right ventricle with PBS, and whole lungs were then harvested and dissected into approximately 1-mm pieces. Single-cell suspensions were prepared with 2 mL of dispase containing collagenase (2 μg/mL) and DNase (50 μg/mL) for 30 min17. After red blood cell lysis, the digested lungs were resuspended in PBS and sequentially filtered through 70-μm filters. Each single-cell suspension was divided into three parts to analyze the number of cytotoxic T lymphocytes (CTL: CD3+CD4-CD8+), regulatory T cells (Treg: CD4+CD25+Foxp3+), and the M1 (CD11b+F4/80+CD206–) and M2 (CD11b+F4/80+CD206+) cells in the lung. The cells were incubated with saturated concentrations of FITC-, PE-, and/or PE-cy5-conjugated mAb against CD3, CD4, CD8, CD25, Foxp3, CD11b, F4/80, or CD206 (diluted 1:50 or 1:100). Isotype-matched mAbs were used in the control samples. CD4+ and CD8+ cells were gated from CD3+ cells. CD4+CD25+ Tregs were gated from Foxp3+ cells. M1 and M2 cells were gated from CD11b+ cells. The data were analyzed using CellQuest software.
ELISA for cytokines in lung tissue
The right lung lobes were lysed in PBS supplemented with a complete protease inhibitor cocktail. The lung tissue homogenate was diluted with lysis buffer to a final protein concentration of 500 μg of protein per mL. The concentrations of IFN-γ and TGF-β1 in the lung were detected using ELISA kits in accordance with the manufacturer's instructions.
Statistical analysis
Differences between groups were assessed by ANOVA. Survival curves were compared by the log-rank test. Our results are presented as the means±standard error (SEM). P values < 0.05 were considered statistically significant.
Results
TLR2-neutralizing antibody and CpG ODN act in synergy to suppress metastasis and improve the survival rates of mice
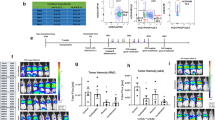
Our group and others have previously reported that blocking TLR2 activity attenuates pulmonary metastasis by inhibiting the immunosuppressive tissue environment12, 13. We wondered if a combination of a TLR2-neutralizing antibody with a TLR9 agonist CpG ODN would exert increased inhibition of tumor metastasis. Lung metastasis was evaluated in mice 3 weeks after tail vein injections with B16 melanoma cells. The number of metastatic nodules in the lungs of mice in the combination treatment group was notably reduced in comparison with the mice treated with the CpG ODN or the TLR2-neutralizing antibody alone upon both visual (Figure 1A) and numerical analysis (Figure 1B). As shown in Figure 1B, the number of metastatic nodes in the lungs of mice treated with the CpG ODN plus the TLR2-neutralizing antibody was markedly attenuated compared with that of the IgG plus CpG ODN control group, the CpG ODN group and the TLR2-neutralizing antibody group. The number of metastatic nodes in the lungs of mice treated with either the CpG ODN or the TLR2-neutralizing antibody was also found to be significantly lower than that of the PBS group and the IgG plus CpG ODN control group. Consistent with these data, the average weight indices of the lungs of PBS-treated mice were markedly increased (15.3‰±1.8‰ vs 7.4‰±0.2‰, P<0.01), whereas the application of CpG ODN resulted in markedly decreased lung weight indices (11.6‰±0.7‰ vs 15.3‰±1.8‰, P<0.05). Further, the application of the TLR2-neutralizing antibody alone (10.5‰±0.6‰) or in combination with CpG ODN (7.8‰±0.4‰) efficiently attenuated the lung weight indicesas compared with the PBS group, the IgG plus CpG control group and the CpG ODN group. The average lung index of the mice that received combination therapy was found to be significantly lower than that of the anti-TLR2 group.
Blocking TLR2 and activating TLR9 markedly suppresses pulmonary metastasis of B16-F10 melanoma cells. (A) Representative lung samples. (B) The metastatic nodules were counted and data presented as the mean±SEM. n=15. (C) The pulmonary weight index of mice in the indicated treatment groups. Data are presented as mean±SEM. n=15 mice per group. (D) Kaplan-Meier graph representing the cumulative survival of mice in the indicated treatment groups. The data were analyzed using Kaplan-Meier survival analysis (n=15 per group). cP<0.01 vs sham. eP<0.05, fP<0.01 vs PBS. iP<0.01 vs anti-TLR2 plus CpG.
To compare the anti-metastatic efficacy of the CpG ODN, the TLR2-neutralizing antibody, and the combination of the CpG ODN and the TLR2-neutralizing antibody, animals in the individually treated groups were monitored for survival time after inoculation with B16 melanoma cells. As shown in Figure 1D, 50% of B16-bearing mice treated with PBS or IgG plus the CpG ODN died by the 29th day after tumor cell inoculation, and all died by the 35th or 36th day. The treatment of B16-bearing mice treated with the TLR2-neutralizing antibody increased the animals' survival rates (50% survival on the 35th day, 0% survival on the 42th day). For the mice treated with the CpG ODN alone, the survival rates decreased to 50% on the 32th day and to 0% on the 40th day. However, the treatment of B16-bearing mice with the CpG ODN plus the anti-TLR2 antibody markedly increased the survival rates and prolonged the survival time; 50% of the animals survived to the 38th day, and 45% of the mice in this group survived until the 48th day. Therefore, the combination of the TLR2-neutralizing antibody with the TLR9 agonist CpG ODN markedly improved the inhibition of pulmonary metastasis of B16F10 cells as compared with either individual treatment alone.
TLR2-neutralizing antibody and CpG ODN induce a synergistic cytotoxicity to B16 melanoma cells
To provide a biological explanation for the superior anti-metastatic effects of the combination regimen, we examined the cytotoxicity of immune cells in TDLNs from the mice treated with the single or combination regimens. Minimal cytotoxicity was detected in the PBS-treated, B16-bearing mice. The treatment of mice with the TLR2-neutralizing antibody or with the CpG ODN alone induced a moderate increase in the cytotoxicity of TDLN immune cells. A markedly enhanced cytotoxicity against B16F10 melanoma cells was detected in the TDLN immune cells of the mice treated with the combination regimen, indicating that the combination regimen induces synergistically enhanced tumor cytolysis activity (Figure 2A). We then examined which immune cells induced the cytotoxicity in these animals. Inoculation of tumor cells resulted in decreases in the numbers of NK cells, the ratios of CD8+/CD4+ and the ratios of Th1/Th2 T cells in the mouse spleens. In comparison to the PBS treatment, the combination regimen and the CpG ODN treatment alone resulted in a 2- or 3-fold increase in the percentage of NK cells and CD8+ T cells in the spleen, respectively. However, compared to the PBS treatment, the treatment of mice with the TLR2-neutralizing antibody alone resulted in only a moderate increase in the percentage of NK cells (6.0%±0.4% vs 9.7%±1.2%, P<0.05) and CD8+ T cells (6.1%±1.0% vs 9.6%±0.9%, P<0.05) in the spleen (Figure 2B, 2C). However, treatment with the TLR2-neutralizing antibody alone or the combination regimen suppressed the proportion of tumor cell-induced CD4+ T cells, while treatment with the CpG ODN alone enhanced the proportion of tumor cell-induced CD4+ T cells (54.3%±7.9% vs 32.7%±2.6%, P<0.05). Therefore, the combination regimen synergistically increased the ratio of CD8/CD4 T cells in the spleen (Figure 2C).
The combinational treatment increases the cytotoxicity of lymphocytes in TDLNs and increases the CD8/CD4 ratio in spleen. (A) Lymphocytes from B16 bearing mice showed the enhanced cytotoxicity after the combined treatment. Lymphocytes from TDLNs of mice with established B16 metastatic nodes were collected for evaluating the lystic activity to B16F10 cells in vitro. Data are presented as the mean ± SEM of 5 mice per group in triplicates. (B–E) The ratios of immune cells in spleen were regulated by the indicated immunotherapy. Splenocytes were isolated and collected for evaluating the amount of NK1.1+ cells (B), the ratio of CD3+/CD8+ and CD3+/CD4+ T cells (C), the ratio of CD4+/IFN-γ+ and CD4+/IL-4+ T cells (D), and the percentage of CD4+/CD25+ regulatory T cells (E). Data are presented as the mean±SEM. of 5 mice per group in triplicates. bP<0.05, cP<0.01 vs sham. eP<0.05, fP<0.01 vs PBS. iP<0.01 vs anti-TLR2 plus CpG.
Why did the CpG ODN treatment alone fail to induce an identical degree of tumor cell cytotoxicity as the combination treatment? We suspected that some fraction of the CD4+ T cells induced by the CpG ODN suppressed the cytotoxicity of the NK cells and the CD8+ T cells. Indeed, the CpG ODN treatment and the combination treatment decreased the frequency of Th2 cells induced by splenic tumor cells (Figure 2D). Treg cells represent a fraction of CD4+ T cells that play a significant role in suppressing anti-tumor immunity. Inoculation of tumor cells resulted in a significant increase in the numbers of Tregs in the spleen. The anti-TLR2 antibody treatment alone and the combination therapy both suppressed the numbers of Tregs. However, the number of splenic Tregs in CpG ODN-treated mice was much higher than that of the anti-TLR2 antibody-treated or the combination regimen-treated mice (Figure 2E). These data indicate that the combination regimen produced greater tumor cell cytotoxicity compared with that of either single agent alone by inducing more NK and CD8+ T cells while simultaneously reducing the numbers of Th2 cells and Tregs in the spleen.
Treatment with TLR2-neutralizing antibody plus CpG ODN enhances the antitumor immunity and reduces immune sup-pression in the tumor microenvironment
To find whether the differential immune responses induced by the combination regimen or by either agent alone was responsible for the differences in metastasis suppression, we examined the lung infiltration of immune cells in animals treated with different agents. A suppressed immune response was observed in the lung tissues of the PBS-treated B16-bearing mice, with decreased infiltration of CD3+CD8+ T cells and M1 cells and increased infiltration of M2 cells and Treg cells (Figure 3A–3D). There was an increase in the lung infiltration of M1 cells, a decrease in the infiltration of M2 cells and no change in the infiltration of CTL (2.47%±0.68% vs 1.73%± 0.28%, P>0.05) and Treg cells (3.35%±0.51% vs 4.25%±0.19%, P>0.05) in the CpG ODN treated mice compared to the PBS-treated mice. Treatment with the TLR2-neutralizing antibody alone or in combination with the CpG ODN significantly increased the lung infiltration of CTL and M1 cells (Figure 3A, 3B) and markedly decreased the infiltration of Treg and M2 cells (Figure 3C, 3D) as compared with the PBS treatment.
The combined therapy increases the infiltration of CTL and M1 cells and decreases the recruitment of M2 and Treg cells in lung tissues. Lung single-cell suspensions were obtained as indicated in the Methods. The number of CTL (A), M1 (B), M2 (C), and Treg (D) cells was analyzed by FCM. Data are presented as mean±SEM (n=5 mice per group). bP<0.05, cP<0.01 vs sham. eP<0.05, fP<0.01 vs PBS. hP<0.05, iP<0.01 vs anti-TLR2 plus CpG.
A number of studies indicate that IFN-γ can induce anti-tumor activity through STAT1 activation and autophagy induction14, 18, 19. By contrast, TGF-β1, IDO and COX2 are immunosuppressive factors that can promote tumor progression through the activation of Treg cells and suppression of T cell proliferation and responses20. As shown in Figure 4, there was a significant decrease in the expression of IFN-γ and a significant increase in the expression of TGF-β1, IDO and COX2 in the lung tissues of PBS-treated B16-bearing mice compared to normal mice. Treatment of mice with the CpG ODN plus the TLR2-neutralizing antibody (98.5±22.6 pg/mL), the CpG ODN alone (159.6±33.1 pg/mL) or the TLR2-neutralizing antibody alone (80.2±9.5 pg/mL) resulted in a significant increase in the expression of IFN-γ compared to PBS treatment (41.6±5.1 pg/mL) (Figure 4A). The treatment of mice with CpG ODN or the TLR2-neutralizing antibody alone did not result in any change in the expression of TGF-β1. However, the CpG ODN plus anti-TLR2 combination treatment (262.4±63.6 pg/mL) significantly reduced the expression of TGF-β1 in the lung tissues compared with the PBS treatment (668.2±112 pg/mL) and CpG ODN treatment (596.3±72.3 pg/mL) (Figure 4B). Furthermore, the expression of IDO and COX2 in the lung tissues from mice treated with the CpG ODN plus the anti-TLR2 antibody was markedly decreased compared with the PBS-treated, CpG ODN-treated and anti-TLR2-treated mice (Figure 4C and 4D). These data suggest that the combination regimen produces a synergistic anti-metastatic effect compared to treatment with the CpG ODN or the anti-TLR2 antibody alone by increasing anti-tumor factors and reducing pro-tumor factors in lung tissues.
The combined treatment augments the expression of IFN-γ and attenuates the expression of TGF-β1, IDO, and COX2 in the lung tissues. Mice were sacrificed 3 weeks after B16 melanoma cells injection. (A−B) The expression of antitumor cytokines IFNγ and immunosuppressive factor TGF-β was detected in lung homogenates from mice using ELISA kits. Data are the mean±SEM. n=5. (C−D) The expression of IDO and COX2 was determined by immunohistochemistry. The IOD was analyzed by Image-Pro Plus image analysis software. The final IOD represents averages from 12 non-overlapping images of each lung specimen. Data are mean±SEM (n=6). cP<0.01 vs sham. eP<0.05, fP<0.01 vs PBS. hP<0.05, iP<0.01 vs anti-TLR2 plus CpG.
Treatment with the TLR2-neutralizing antibody plus CpG ODN suppresses the spontaneous metastasis of Lewis lung carcinoma
To verify the antitumor effect of the TLR2-neutralizing antibody plus the CpG ODN, we examined whether this regimen could suppress the growth and spontaneous metastasis of Lewis lung carcinoma cells. Lewis lung carcinoma cells were transplanted into the right flanks of the mice. The chemotherapeutic agent CTX, used as positive control, inhibited the growth (1.5±0.2 vs 5.3±0.6 g, P<0.001) and spontaneous metastasis (5.6±1.7 vs 24.6±3.5, P<0.001) of the Lewis lung carcinoma cells (Figure 5A). As shown in Figure 5B, the average weight of the primary tumor 24 days post-inoculation was 5.3±0.6 g in the PBS-treated mice, 5.5±0.5 g in the isotype IgG plus CpG ODN control-treated mice, and 4.7±0.5 g in the mice treated with the TLR2-neutralizing antibody plus the CpG ODN (P>0.05) (Figure 5B). However, the number of metastatic nodes in the lungs of the mice treated with the TLR2-neutralizing antibody plus CpG ODN was markedly decreased by 53.3%±11.3% as compared with the PBS-treated and isotype IgG plus CpG ODN control-treated mice (Figure 5C). The lung index was also reduced in the combination-treated mice (Figure 5D). These results suggest that combining the TLR2-neutralizing antibody with the CpG ODN is not sufficient to attenuate the growth of established tumor but is efficient in the attenuation of tumor metastasis.
The combined therapy does not suppress the growth of primary tumor but attenuates the second pulmonary metastasis. (A) Primary tumor growth was monitored by counting the size of the external tumor lump every 4th day. (B) The tumor weight was monitored after mice were sacrificed. (C) The metastatic nodules were counted and data presented as the mean±SEM (n=15). (D) The pulmonary weight of mice in the indicated treatment groups. bP<0.05, cP<0.01 vs sham. eP<0.05, fP<0.01 vs PBS. hP<0.01, iP<0.01 vs anti-TLR2 plus CpG.
Discussion
The major finding of this study is that a rational combination of a TLR2-neutralizating antibody, which eliminates the inhibitory immune factors from tumor tissue, and the TLR9 agonist CpG ODN, which can increase the anti-tumor stimulatory factors, is more effective in inhibiting experimental and spontaneous metastasis than either agent alone. Indeed, the synergistic anti-metastatic effects of the two agents are the result of enhanced immune cytotoxicity against the tumor cells. This is reflected by numerous systemic and local changes in the immune responses: increases in the frequency of anti-tumor effector cell infiltration (such as NK, CTL, and M1 cells); decreases in the frequency of pro-tumor suppressor cell infiltration (such as Tregs, M2 cells); the enhanced expression of IFN-γ; and the downregulation of immunosuppressive factors including TGF-β1, IDO, and COX2. Treatment with either agent alone also induced a similar pattern of changes in the immune response, but the magnitudes observed were significantly less than those of the combination regimen. However, the combination regimen does not suppress tumor growth. This result may be due to the following: 1) the importance of TLR2 and TLR9 on host immune cells and tumor cells for positively modulating metastatic behavior compared to their minimal influence on primary subcutaneously implanted tumor growth12, 13, 21, 22; 2) the failure of the combination regimen to promote sufficient immune cell infiltration into the primary tumor to produce a potent cytotoxic suppression of tumor growth due to the anatomical site of administration23; 3) the inability of the therapeutic administration of the combination regimen to overcome the immunosuppressive barrier after the suitable microenvironment for tumor growth has been established14.
The TLR9 agonist CpG ODN is a promising anti-cancer immunotherapy based on its ability to safely stimulate Th1-dominant innate and adaptive immunity in humans7. Nevertheless, tumors employ multiple means of suppressing or evading antitumor immunity24 and cannot be overcome by the CpG ODN treatment alone. For example, the CpG ODN is a potent inducer of IL-10 from DCs and has been found to protect Streptozocin-induced autoimmune diabetes by the induction of IDO and Tregs25. Indeed, administration of the CpG ODN in the current study increased the frequency of NK and CTL cell infiltration, IFNγ secretion, and M1 cell differentiation but did not suppress the number of Tregs in the spleen. Further, the TLR9 agonist CpG ODN increased the expression of TGF-β1, IDO, and COX-2 and promoted the infiltration of Treg cells into the tumor microenvironment after tumor inoculation. The Tregs, other immune-suppressive cells, cytokines and enzymes subsequently counteract the initiation and perpetuation of antitumor cytotoxicity either by direct cell contact or by secreting suppressive cytokines. Thus, the cytotoxicity of the immune cells from CpG ODN-treated B16-bearing mice is diminished even though the numbers of NK and CTL cells are increased in the spleen. Xiong Z et al recently demonstrated that CpG ODN immunotherapy in a breast cancer model prevents but fails to entirely eradicate established brain metastases despite a significant increase in brain-infiltrating T and natural killer cells in treated mice relative to saline controls26. These findings indicate that CpG ODN treatment alone can stimulate anti-tumor effectors but fails to reverse the immunosuppressive responses in the tumor microenvironment. Therefore, the anti-metastatic activity of CpG ODN monotherapy is limited.
TLR2 is an unique member of the TLR family because it triggers an immunosuppressive response in vivo27. Activation of TLR2 promotes IL-10 production by innate immune cells in vitro and results in the proliferation of Tregs in vitro and in vivo28. Treg numbers in the circulation of TLR2-deficient mice are reduced compared with their WT littermate controls10. Furthermore, the activation of the TLR2 signal may be crucial for tumor cells to escape killing by the host29. Huang et al recently found that activation of TLR2 on cancer cells by Listeria monocytogenes promotes tumor growth30, while Kim et al reported that activation of TLR2 by an extracellular matrix proteoglycan versican of Lewis lung carcinoma stimulates pro-tumor inflammation and metastasis13. We recently demonstrated that the level of TLR2 expressed on B16F10 melanoma cells determines their invasive activity in response to the endogenous factors released from tumor cells12. However, contradictory observations by others indicate that TLR2 activation is able to induce inflammatory cytokines and activate NK cells in vitro and in vivo and protect mice from tumor development and progression31, 32. In the current study, we find that blocking TLR2 effectively reverses the immunosuppressive characteristics of the microenvironment by suppressing the expression of TGF-β1, IDO, and COX2 and attenuating the infiltration of M2 and Tregs cells. This scenario is an example of how immunotherapy is able to break self-tolerance by depleting immunosuppressive factors in the tumor microenvironment to generate effective anti-tumor immunity.
In summary, our studies indicate that developing an optimal immunotherapeutic strategy in which combining an immune stimulator with an agent eliminating inhibitory immune factors is critical for obtaining a desired therapeutic effect against tumor metastasis. Our studies provide novel insights into the development of the rational immunotherapeutic strategies against cancer.
Author contribution
Jun YAN and Fang HUA designed and performed most of the experiments, analyzed and interpreted the data and contributed to writing the manuscript; Hong-zheng YANG and Han-zhi LIU performed and analyzed individual experiments; and Zhuo-wei HU conceived the project, designed and interpreted experiments and modified and edited the manuscript.
References
King J, Waxman J, Stauss H . Advances in tumour immunotherapy. Qjm 2008; 101: 675–83.
Lasaro MO, Ertl HC . Targeting inhibitory pathways in cancer immunotherapy. Curr Opin Immunol 2010; 22: 385–90.
Curiel TJ . Tregs and rethinking cancer immunotherapy. J Clin Invest 2007; 117: 1167–74.
Rüttinger D, Winter H, van den Engel NK, Hatz R, Jauch KW, Fox BA, et al. Immunotherapy of cancer: key findings and commentary on the Third Tegernsee Conference. The Oncologist 2010; 15: 112–8.
Kawai T, Akira S . The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat immunol 2010; 11: 373–84.
de Visser KE, Eichten A, Coussens LM . Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006; 6: 24–37.
Krieg AM . Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117: 1184–94.
Schmidt C . Clinical setbacks for toll-like receptor 9 agonists in cancer. Nat Biotechnol 2007; 25: 825–6.
Kopfstein L, Christofori G . Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci 2006; 63: 449–68.
Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H . TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 2008; 27: 218–24.
Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR . Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 2006; 116: 2022–32.
Yang HZ, Cui B, Liu HZ, Mi S, Yan J, Yan HM, et al. Blocking TLR2 activity attenuates pulmonary metastases of tumor. PloS One 2009; 4: e6520.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009; 457: 102–6.
Yan J, Wang ZY, Yang HZ, Liu HZ, Mi S, Lv XX, et al. Timing is critical for an effective anti-metastatic immunotherapy: the decisive role of IFNgamma/STAT1-mediated activation of autophagy. PloS One 2011; 6: e24705.
Russell JH, Ley TJ . Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 2002; 20: 323–70.
Molavi O, Ma Z, Hamdy S, Lai R, Lavasanifar A, Samuel J . Synergistic antitumor effects of CpG oligodeoxynucleotide and STAT3 inhibitory agent JSI-124 in a mouse melanoma tumor model. Immunol Cell Biol 2008; 86: 506–14.
Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE . The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol 1994; 55: 35–42.
Martini M, Testi MG, Pasetto M, Picchio MC, Innamorati G, Mazzocco M . IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 2010; 28: 3548–57.
Dunn GP, Koebel CM, Schreiber RD . Interferons, immunity and cancer immunoediting. Nat Rev 2006; 6: 836–48.
Muller AJ, Scherle PA . Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer 2006; 6: 613–25.
Xie W, Wang Y, Huang Y, Yang H, Wang J, Hu Z . Toll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun 2009; 379: 1027–32.
Wang C, Cao S, Yan Y, Ying Q, Jiang T, Xu K, et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer 2010; 10: 415.
Nierkens S, den Brok MH, Roelofsen T, Wagenaars JA, Figdor CG, Ruers TJ, et al. Route of administration of the TLR9 agonist CpG critically determines the efficacy of cancer immunotherapy in mice. PloS One 2009; 4: e8368.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51.
Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol 2009; 183: 6303–12.
Xiong Z, Gharagozlou S, Vengco I, Chen W, Ohlfest JR . Effective CpG immunotherapy of breast carcinoma prevents but fails to eradicate established brain metastasis. Clin Cancer Res 2008; 14: 5484–93.
Netea MG, Van der Meer JW, Kullberg BJ . Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol 2004; 12: 484–8.
Yamazaki S, Okada K, Maruyama A, Matsumoto M, Yagita H, Seya T . TLR2-dependent induction of IL-10 and Foxp3+ CD25+ CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PloS One 2011; 6: e18833.
Rüttinger D, Winter H, van den Engel NK, Hatz R, Jauch KW, Fox BA, et al. Immunotherapy of cancer: key findings and commentary on the third Tegernsee conference. Oncologist 2010; 15: 112–8.
Xiong Z, Gharagozlou S, Vengco I, Chen W, Ohlfest JR . Effective CpG immunotherapy of breast carcinoma prevents but fails to eradicate established brain metastasis. Clin Cancer Res 2008; 14: 5484–93.
Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res 2007; 67: 4346–52.
Lu H, Yang Y, Gad E, Wenner CA, Chang A, Larson ER, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin Cancer Res 2010; 17: 67–76.
Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PloS One 2010; 5: e13027.
Acknowledgements
This study was supported by grants from the Creation of Major New Drugs (2009ZX09301-003-13; 2009ZX09301-003-9-1), the National Natural Science Foundation of China (30973557), and the PhD. Programs Foundation of the Ministry of Education of China (20070023035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, J., Hua, F., Liu, Hz. et al. Simultaneous TLR2 inhibition and TLR9 activation synergistically suppress tumor metastasis in mice. Acta Pharmacol Sin 33, 503–512 (2012). https://doi.org/10.1038/aps.2011.193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.193
Keywords
This article is cited by
-
IR-780-loaded polymeric micelles enhance the efficacy of photothermal therapy in treating breast cancer lymphatic metastasis in mice
Acta Pharmacologica Sinica (2018)
-
Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy
Cancer Immunology, Immunotherapy (2014)