Abstract
Aim:
To investigate the neuroprotective effect of glycyrrhizin (Gly) against the ischemic injury of rat spinal cord and the possible role of the nuclear protein high-mobility group box 1 (HMGB1) in the process.
Methods:
Male Sprague-Dawley rats were subjected to 45 min aortic occlusion to induce transient lumbar spinal cord ischemia. The motor functions of the animals were assessed according to the modified Tarlov scale. The animals were sacrificed 72 h after reperfusion and the lumbar spinal cord segment (L2–L4) was taken out for histopathological examination and Western blotting analysis. Serum inflammatory cytokine and HMGB1 levels were analyzed using ELISA.
Results:
Gly (6 mg/kg) administered intravenously 30 min before inducing the transient lumbar spinal cord ischemia significantly improved the hind-limb motor function scores, and reduced the number of apoptotic neurons, which was accompanied by reduced levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in the plasma and injured spinal cord. Moreover, the serum HMGB1 level correlated well with the serum TNF-α, IL-1β and IL-6 levels during the time period of reperfusion.
Conclusion:
The results suggest that Gly can attenuate the transient spinal cord ischemic injury in rats via reducing inflammatory cytokines and inhibiting the release of HMGB1.
Similar content being viewed by others
Introduction
Ischemic spinal cord injury (ISCI) is a serious complication that can result from thoracoabdominal aortic surgery and can cause paraplegia in 2% to 18% of patients1, 2. In a recent report, only 5% of 127 patients who underwent clamp/sew surgery developed paraplegia3; paraplegia caused by ISCI remains a problem that should be solved. Multiple studies have suggested that calcium overload, inflammatory processes, free radical production, platelet aggregation, neutrophil accumulation and adhesion following ischemia might contribute to the neuronal damage that was observed in patients with ISCI4, 5. However, the cellular and molecular mechanisms of ischemic spinal cord injury are not fully understood. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) are key proinflammatory cytokines that play important functions in the central nervous system during inflammatory injury. Besides causing direct damage to cell membranes, free oxygen radicals activate the accumulation of neutrophils and stimulate various types of cells to produce TNF-α and IL-1β6. These cytokines further contribute to the production of other cytokines and to the expression of endothelial leukocyte adhesion factor-1, ultimately leading to endothelial cell damage and spinal cord ischemia7, 8. Recent studies have shown that the high-mobility group box 1 (HMGB1) protein, an abundant nuclear protein that acts as an architectural chromatin binding factor, can be passively released by necrotic or damaged cells and serves as a signaling molecule that is involved in acute and chronic inflammation9, 10. A wealth of evidence indicates that HMGB1 is massively released during the excitotoxicity-induced, acute damaging process in the post-ischemic brain, where it triggers inflammatory processes, and suggests that HMGB1 acts as a novel mediator that links excitotoxicity-induced acute damage and subsequent inflammatory processes in the post-ischemic brain11, 12, 13.
Along these lines, we have recently recognized glycyrrhizin (Gly), a natural triterpene glycoconjugate that is derived from the root of licorice (Glycyrrhiza glabra), as an additional HMGB1 inhibitor. Gly binds directly to both HMG boxes in HMGB1, thereby inhibiting its chemoattractant functions in fibroblasts and smooth muscle cells14, 15. Of note, Gly is a natural compound that is commonly used in Japan to treat patients with chronic hepatitis16; however, no study has been designed to examine its use in preventing ISCI.
The current study was designed to investigate the protective efficacy and dose-response relationship of Gly against the neurologic and histopathological outcomes of spinal cord ischemia and reperfusion injury that are related to aortic occlusion in rats, and to determine whether HMGB1 plays a pathogenetic role in ischemic spinal cord injury. First, we injected ischemic rats with either glycyrrhizin or a placebo. Second, we monitored the concentration of HMGB1, TNF-α, IL-1β, and IL-6 in the plasma of the rats. Third, we detected the expression of HMGB1 and cell death within the ischemic spinal cords of those rats.
Materials and methods
Animals and groups
Male Sprague-Dawley rats weighing 300–350 g were obtained from the Experimental Animal Center of Sichuan University (Chengdu, China) and were allowed free access to laboratory chow and tap water in day-night regulated quarters at 25 °C.
Rats were randomized into the following three experimental groups, each consisting of 15 animals: (i) ISCI rats that were pretreated with saline (NS group); (ii) ISCI rats that were pretreated with Gly (Minophagen Pharmaceutical Co, Tokyo, Japan) at a dose of 6 mg/kg (Gly group); and (iii) healthy, control, sham-operated rats (Sham group). In the pretreatment groups, glycyrrhizin or saline was administered intravenously via the tail vein 30 min before the induction of ischemia/reperfusion (I/R) ISCI.
Experimental I/R spinal cord injury
The detailed surgical method for transient lumbar spinal cord ischemia has been described previously17. Briefly, rats were initially anesthetized with intramuscular ketamine (50 mg/kg) and then by a half dose of ketamine, as required for the procedure. During the surgery, body temperature was monitored using a rectal probe and was maintained at 35.5–37.5 °C with a heat lamp. During the procedure, an intravenous catheter was placed into the tail vein, and 0.9% NaCl was infused. Cefazolin was injected intravenously at a single dose of 10 mg/kg immediately before the surgery to prevent infection. To monitor proximal and distal aortal blood pressures, catheters were surgically placed into the left common carotid artery and the left femoral artery, respectively. The abdominal aorta was accessed through a midline laparotomy, and animals in the sham group were subjected to laparotomy without aortic occlusion. For the other groups, animals were subjected to 45 min of cross clamping, where vascular clamps were placed under the left renal vein and above the bifurcation in the aorta. Each rat received 150 IU/kg of heparin before aortic occlusion, the aortic clamps were removed after 45 min, and the abdomen was closed appropriately. Animals were allowed to recover in a plastic box at 28 °C for 3 h and were subsequently placed in their cages with free access to food and water. The Crede maneuver was used twice daily to empty the urinary bladders of paraplegic animals. Animals that never recovered completely from the surgery and died within 24 h after reperfusion were excluded from the analyses.
Serum detection
Blood samples (0.4 mL) were collected from the femoral vein at 0 h, 0.5 h, 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h after reperfusion. Serum was isolated from the blood after centrifugation at 1500 r/min for 15 min and was frozen at -80 °C until enzyme-linked immunosorbent assay (ELISA) analyses were performed. HMGB1 concentrations and the levels of inflammatory mediators (TNF-α, IL-1β, and IL-6) in the serum samples were quantified using specific ELISA kits for rats according to the manufacturers' instructions (Biosource International Inc, Camarillo, CA, USA).
Neurological assessment
The motor functions of the rats were assessed at 24 h, 48 h, and 72 h after the procedure using the following modified Tarlov scale6, 17, 18: 0, no voluntary movement (complete paraplegia); 1, perceptible movement at the joint; 2, good joint mobility but unable to stand; 3, ability to stand but unable to walk; 4, weak walking; 5, complete recovery.
Spinal cord HMGB1 contents
The lumbar enlargements of the spinal cords of rats that were killed at the completion of behavioral testing were removed. Five samples from every group were stored at -80 °C until Western blotting analyses were performed. Briefly, frozen samples were mechanically lysed in a homogenization buffer on ice. The lysates were centrifuged at 12 000 r/min for 20 min at 4 °C, and the protein concentrations were estimated using a BCA protein assay kit (Jiancheng Bioengineering Institute, Nanjing, China). Each sample was adjusted to a final total protein concentration of 5 μg/μL in 4×sample buffer, heated at 95 °C for 10 min, and then stored at -20 °C. Protein samples (50 μg per lane) were loaded into a 12% SDS-PAGE gel and run at 100 V for 120 min in running buffer. Proteins were then transferred from the gel to a PVDF membrane at 250 mA for 90 min using transfer buffer. The membrane was blocked with 5% skimmed milk for 2 h at room temperature and incubated overnight at 4 °C with primary antibody directed against HMGB1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:500. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (diluted in 1:6000, Sigma-Aldrich Inc, St Louis, MO, USA) was used as a loading control. After 6×10 min rinses with PBS/Tween, the membrane was incubated in the appropriate HRP-conjugated secondary antibody (diluted 1:1000 in PBST) for 2 h. The blotted protein bands were visualized using enhanced chemiluminescence (ECL) Western blotting detection reagents (Millipore, Billerica, MA, USA), and the blots were exposed to X-ray film. Developed films were digitized using an Epson Perfection 2480 scanner (Seiko, Nagano, Japan), and optical densities were obtained using Glyko Bandscan software (Glyko, Novato, CA, USA). All experiments were repeated at least three times.
Histological examination
The remnant samples (n=10 for each group) were fixed in 10% formalin, embedded in paraffin and cut to a thickness of 6 μm, with a routine follow-up procedure. An observer who was uninformed of the experimental conditions of the animals recorded the data.
Coronal sections were stained with hematoxylin and eosin (HE) for light microscopic examination. Changes in rat motor neurons caused by ischemia were identified to be shrunken cellular bodies, a disappearance of Nissl granules, an intensely eosinophilic cytoplasm and triangular and pyknotic nuclei. The remaining normal neurons in the ischemic ventral spinal cord of each animal, as judged by their morphological appearance, were counted in three sections that were selected randomly from the rostral, middle, and caudal levels of the L4 segment and then averaged. The numbers of normal neurons per section of the anterior spinal cords of the rats (anterior to an imaginary line drawn through the central canal, which was perpendicular to the vertical axis) were compared between three groups.
TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling) reactions were applied to identify cells with fragmented DNA according to the instruction manual of a commercial TUNEL kit (Roche, Basel, Switzerland). Cell viability was assessed by visual inspection of damaged cells that had been stained with TUNEL, and data are presented as the number of TUNEL-positive cells from three sections of the same animal.
Statistical analysis
All data, except neurologic scoring, were presented as the mean±SEM (standard error of mean), which was calculated using SPSS (Statistical Package for the Social Sciences) 12.0 software (SPSS Inc, Chicago, IL, USA). The Mann-Whitney U-test was used to compare the behavior and activity score among groups, and the concentrations of serum HMGB1 and inflammatory mediators were analyzed using two-way repeated-measures (time and group) analysis of variance followed by the post hoc Student-Newman-Keuls test. Correlations between HMGB1 levels and concentrations of inflammatory mediators were analyzed using Spearman's rank correlation test, and the number of normal neurons and TUNEL-positive motor neurons in the anterior spinal cord were analyzed using the Kruskal-Wallis test followed by the Mann-Whitney U-test with the Bonferroni correction. The P<0.05 level of probability was used as the criteria for significance.
Results
Serum HMGB1 concentrations
As shown in Figure 1, the HMGB1 serum concentrations in the sham animals were unchanged during the period of the experimental procedure. However, the concentrations of HMGB1 in the serum of the NS and Gly groups significantly increased 2 h after reperfusion, when compared to pre-ischemia levels, and the concentrations remained at higher levels thereafter (P< 0.05). Furthermore, HMGB1 serum concentrations in the animals that were treated with Gly were significantly lower than in those of the NS group from 2 h to 72 h after reperfusion (P< 0.05).
Time course of serum HMGB1 concentrations. The serum HMGB1 levels were significantly increased after spinal cord ischemia-reperfusion compared with that of preischemia (P<0.05), whereas they were significantly decreased in the animals treated by Gly compared with that of animals in NS group (P<0.05). Data are means±SEM. n=15 for each group. bP<0.05 vs NS group.
Concentrations of inflammatory cytokines in the serum
The concentrations of TNF-α, IL-1β, and IL-6 were low in the serum of the rat sham group (Figure 2); however, serum levels of these inflammatory cytokines were greatly induced from 6 h to 72 h after reperfusion in the experimental groups (P<0.05). As shown in Figure 2, Gly administration before I/R resulted in significantly decreased IL-1β, TNF-α, and IL-6 concentrations compared to the NS group (P<0.05). Moreover, the serum HMGB1 contents correlated well with the levels of TNF-α (r=0.947), IL-1β (r=0.906), and IL-6 (r=0.935) at 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h after reperfusion (Figure 3).
Neurologic outcomes
All animals survived until the final neurologic behavior assessments at 24 h, 48 h, and 72 h after reperfusion. The hind-limb motor function scores of the 3 groups at 24 h, 48 h, and 72 h after reperfusion are shown in Table 1. In the NS group, most of the animals developed complete paraplegia of the hind-limbs (grade 1) at 72 h after reperfusion. Importantly, the neurologic statuses of members of the Gly group were significantly improved, compared to those of the NS group, at 24 h, 48 h, and 72 h after reperfusion (P=0.029, 0.001, 0.004, respectively).
Western blotting
The protein levels of HMGB1 were detected by Western blot analysis (Figure 4). The protein was expressed at low levels in the spinal cords of the sham group members; however, the levels of HMGB1 significantly increased in the spinal cords of members of the experimental groups as compared with HMGB1 levels of the sham groups (P=0.006). Furthermore, the protein expression of HMGB1 in the spinal cords of members of the Gly group was significantly lower than that of the NS group (P=0.035).
Histological examination
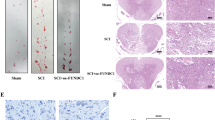
The representative micrographs of HE staining of the ventral horn of the L4 spinal cord segment 72 h after reperfusion are shown in Figure 5A–5C. The number of normal cells in the Gly group was more than that in the NS group (Figure 5D, P=0.019), and TUNEL staining identified a few dead cells in the cord sections of the sham-operated animals (Figure 6A). In the spinal cords of members of the NS group, numerous cells were strongly positive for TUNEL staining (Figure 6B). However, in samples from the Gly group, only a few cells were positive for TUNEL staining (Figure 6C). For quantitative measurement, the number of cells that were positive or negative for TUNEL was recorded for each specimen in a blind fashion. Administration of Gly 30 min before ischemia significantly reduced the total number of dead cells, compared to that of the NS group (Figure 6D, P=0.016). Moreover, the number of dead cells correlated well with the HMGB1 levels in spinal cord tissue at 72 h after reperfusion (Figure 6E, n=15 pairs, r=0.929, P=0.005).
Coronary section of the lumbar spinal cords and quantification of normal motor neurons. (A–C) Representative micrographs of H&E staining in the ventral horn of spinal cord of L4 segments in the Sham, NS and Gly groups at 72 h after reperfusion, respectively (×200). (D) The bar graph showing the quantitative analysis of the number of normal motor neurons in the anterior horn of spinal cord of L4 segments in 3 groups. Data are means±SEM. n=10 for each group. bP<0.05 vs NS group. Scale bars=80 μm.
Representative fluorescence micrographs of TUNEL staining and quantification of apoptotic motor neurons. (A–C) Representative fluorescence micrographs of TUNEL staining in the ventral horn of spinal cord of L4 segments from animals in the sham, NS and Gly groups at 72 h after reperfusion, respectively (×200). (D) Quantitative analysis of the number of TUNEL-positive cells in the anterior horn of spinal cord of L4 segments in three groups. Data are means±SEM. n=10 for each group. bP<0.05 vs NS group. Scale bars=80 μm. (E) The numbers of dead cells correlated well with the HMGB1 levels of spinal cord tissue in the spinal cord at 72 h after reperfusion. n=15 pairs, r=0.929, P=0.005.
Discussion
There is a wealth of evidence to suggest that the systemic inflammatory response that is associated with I/R injury contributes to the morbidity and mortality that is associated with the repair of thoracoabdominal aortic aneurysms19. The principal mechanisms of pharmacological therapy, such as the administration of high doses of the glucocorticoid steroid methylprednisolone that are used in humans, are likely to inhibit posttraumatic lipid peroxidation and inflammatory responses. In this study, we show that Gly significantly attenuated spinal cord I/R injury when administered 30 min before ischemia, and this protection was accompanied by a reduction in serum inflammatory factors and the protein HMGB1.
HMGB1 is a non-histone, nuclear protein with dual functions. Inside cells, HMGB1 binds DNA and plays a role in transcriptional regulation. Outside cells, HMGB1 serves as a late cytokine-like mediator of systemic inflammation20. HMGB1 can activate inflammatory pathways when released from ischemic cells, and studies indicate that HMGB1 acts as an early mediator of inflammation and organ damage in hepatic I/R injury. HMGB1 levels were increased during liver I/R as early as 1 h after reperfusion and then further increased, in a time-dependent manner, up to 24 h. Inhibition of HMGB1 activity with a neutralizing antibody significantly decreased liver damage after I/R, whereas administration of recombinant HMGB1 worsened I/R injury21, 22. Moreover, HMGB1 is massively released extracellularly and plays a cytokine-like function in the postischemic brain11, 12. HMGB1, as a mediator of postischemic brain damage, plays a critical role in the development of brain infarction through the amplification of plural inflammatory responses in the ischemic region and could be an outstandingly suitable target for treatment for this damage23. Intravenous injection of a neutralizing, anti-HMGB1 monoclonal antibody provides a novel therapeutic strategy for ischemic stroke24. In addition, serum HMGB1 levels were significantly elevated in patients with myocardial ischemia and cerebral ischemia, suggesting that systemic HMGB1 levels are elevated in human ischemic disease25. In this study, serum HMGB1 concentrations and levels of IL-β, TNF-α, and IL-6 increased during spinal cord I/R as early as 2 h after reperfusion and in a time-dependent manner up to 72 h. These results indicate that HMGB1 is involved in the proinflammatory stress response to I/R injuries of the spinal cord in a time-dependent manner after spinal cord I/R in rats.
Obviously, inhibition of HMGB1 secretion or release represents a novel and promising strategy for the therapy of I/R injuries23. A growing amount of information implicates a possible responsibility of inflammatory mediators in the pathogenesis of spinal cord injury. In a rat model of traumatic SCI, the tissue level of TNF-α in the spinal cord significantly increased 24 h after injury26. Similarly, in a mouse model of traumatic SCI, TNF-α, and IL-1β were produced almost immediately following injury, and this production was followed by the expression of IL-627. Clinical research has also revealed increased immunoreactivity of TNF-α, IL-1β, and IL-6 in neurons at both early and late phases of trauma in human spinal cord tissues after injury28. In the present study, we demonstrated that serum proinflammatory cytokine levels (TNF-α, IL-1β, and IL-6) significantly increased after spinal cord I/R in rats. These increases were accompanied by elevated HMGB1 concentrations, and by analyzing histopathological specimens, tissue damage to the spinal cord was evident. In the NS group, all three proinflammatory cytokine levels reached significantly higher levels when compared to the sham-operated group, and these elevated levels were relieved by treatment with Gly. Treatment with Gly attenuated serum HMGB1 levels after spinal cord I/R injury when the drug was administered 30 min before ischemia. Moreover, the HMGB1 contents of spinal cord tissue in animals that had been treated with Gly 72 h after reperfusion were found to be significantly lower than those of the controls. To the best of our knowledge, this is the first study to demonstrate a protective effect of Gly that is related to its inhibitory effect on HMGB1 release in spinal cord I/R injuries. We found that I/R upregulated the expression of HMGB1 in injured tissue and the levels of IL-β, TNF-α, and IL-6 in the peripheral blood, which was inhibited by Gly administration. These results suggest that I/R could activate HMGB1, which might play a central role in the inflammatory response that leads to secondary insults after ischemia. Therefore, the therapeutic benefit of pre-I/R Gly administration might be due to its salutary effect on modulating HMGB1.
However, apoptosis has been demonstrated to be an important mechanism of neuron death in the ischemic spinal cord, and to play an important role in delayed paraplegia in the animal model of aortic occlusion29. It is important to note that the TUNEL assay does not distinguish between cell death mechanisms (necrosis or apoptosis); however, this method is useful for detecting damaged cells using light or fluorescence microscopy30. Furthermore, histopathological examinations of the spinal cords in our study revealed that there was significant neuronal loss in both 72-h I/R groups, when compared to the sham-operated groups. In this study, dead cells were detected based on positive TUNEL staining because the fluorescent nucleus developed a granular pattern. We used this method because of its high sensitivity and specific means of identifying DNA fragmentation. As noted, numerous dead cells were observed in the spinal cords of the control animals, and the total number of TUNEL-positive cells was reduced significantly after Gly treatment. The results showed that Gly alleviated cell apoptosis that was induced by spinal cord I/R. In line with this, the animals that were treated with Gly had better neurologic outcomes than those of the NS group. Moreover, at 72 h after reperfusion, the HMGB1 levels in spinal cord tissue from animals that had been treated with Gly were significantly lower than those of the NS group, and these levels correlated well with the numbers of dead cells in the spinal cord 72 h after reperfusion. Together, these results indicate that inhibiting the release of HMGB1 with Gly results in less tissue damage and better functional recovery of neurons. In accordance with our experimental results, the relationship between apoptosis and HMGB1 release in macrophages and other cells was investigated in an in vitro study, and those results indicated that the release of HMGB1 from macrophages correlated with the occurrence of apoptosis, and suggested that these processes reflected common mechanisms and could occur concomitantly31. However, other studies have shown that HMGB1 production occurred downstream of apoptosis in the final common pathway to organ damage in severe sepsis32. Thus, the crosstalk between HMGB1 and apoptosis must be further explored.
Because there are two contradictory pathways for inflammation and apoptosis, it is interesting that Gly influences the two pathways simultaneously. Here, two mechanisms could be considered to cause this result. First, Gly could inhibit inflammation by suppressing HMGB1 expression. HMGB1 has been thought to take part in anti-inflammation because it activates inflammatory responses through multiple pathways, including activating the MAPK pathway and then NF-κB translocation, which triggers inflammatory responses33. These pathways lead to a cascade of inflammatory responses that can cause tissue damage and the release of inflammatory mediators. Secondly, the TUNEL assay is only useful for detecting dead cells, and this method does not distinguish between cell death mechanisms (necrosis or apoptosis)30. Because inflammatory responses can cause tissue damage and even death, Gly could reduce the number of TUNEL-positive cells by suppressing HMGB1 expression in this study. A limitation to our present study is that we did not assay the mechanism of Gly in attenuating cell damage.
In conclusion, our results confirmed that HMGB1 release plays an important role in spinal cord I/R damage34, and we showed the Gly affords strong protection against transient spinal cord I/R injury by reducing inflammatory factors and cell apoptosis. Moreover, this protective effect by Gly is related to the inhibition of HMGB1 release that is induced by spinal cord I/R. These data suggest a new therapeutic possibility for treating ISCI with Gly. Future research should be directed toward developing a better understanding of the crosstalk between HMGB1 and apoptosis, as this ultimately might lead to therapeutic strategies for humans.
Author contribution
Prof Le-shun ZHOU designed the research and revised the manuscript; Gu GONG conducted the research, analyzed the data and wrote the paper; Li-bang YUAN, Ling HU, Wei WU, Liang YIN, and Jing-li HOU helped with portions of the research, and Ying-hai LIU helped write the manuscript.
References
Ronald AK, Marc ES, David MM . Anesthetic consideration for descending thoracic aortic aneurysm repair. Semin Cardiothorac Vasc Anesth 2007; 11: 205–23.
Tabayashi K . Spinal cord protection during thoracoabdominal aneurysm repair. Surg Today 2005; 35: 1–6.
Conrad MF, Ergul EA, Patel VI, Cambria MR, Lamuraglia GM, Simon M, et al. Evolution of operative strategies in open thoracoabdominal aneurysm repair. J Vasc Surg 2011; 53: 1195–201.
Danielisova V, Chavko M . Comparative effects of the N-methyl-D-aspartate antagonist MK-801 and the calcium channel blocker KB-2796 on neurologic and metabolic recovery after spinal cord ischemia. Exp Neurol 1998; 149: 203–8.
Agee JM, Flanagan T, Blackbourne LH, Kron IL, Tribble CG . Reducing postischemic paraplegia using conjugated superoxide dismutase. Ann Thorac Surg 1991; 51: 911–5.
Hirose K, Okajima K, Taoka Y, Uchiba M, Tagami H, Nakano K, et al. Activated protein C reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann Surg 2000; 232: 272–80.
Klebanoff SJ, Vadas MA, Harlan JM, Sparks LH, Gamble JR, Agosti JM, et al. Stimulation of neutrophils by tumor necrosis factor. J Immunol 1986; 136: 4220–5.
Taoka Y, Okajima K, Murakami K, Johno M, Naruo M . Role of neutrophil elastase in compression-induced spinal cord injury in rats. Brain Res 1998; 799: 264–9.
Muller S, Ronfani L, Bianchi ME . Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 2004; 255: 332–43.
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P . HMGB1: guiding immunity from within. Trends Immunol 2005; 26: 381–7.
Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, et al. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 2007; 103: 590–603.
Kim JB, Lim CM, Yu YM, Lee JK . Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res 2008; 86: 1125–31.
Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 2006; 26: 6413–21.
Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997; 79: 1494–500.
Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 2007; 14: 431–41.
van Rossum TG, Vulto AG, Hop WC, Schalm SW . Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol 2001; 96: 2432–7.
Akgun S, Tekeli A, Kurtkaya O, Civelek A, Isbir SC, Ak K, et al. Neuroprotective effects of FK-506, L-carnitine and azathioprine on spinal cord ischemia-reperfusion injury. Eur J Cardiothorac Surg 2004; 25: 105–10.
Nakayama T, Harada N, Asano M, Nomura N, Saito T, Mishima A, et al. Atrial natriuretic peptide reduces ischemia/reperfusion-induced spinal cord injury in rats by enhancing sensory neuron activation. J Pharmacol Exp Ther 2007; 322: 582–90.
Fiane AE, Videm V, Lingaas PS, Heggelund L, Nielsen EW, Geiran OR, et al. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation 2003; 108: 849–56.
Yamada S, Maruyama I . HMGB1, a novel inflammatory cytokine. Clin Chim Acta 2007; 375: 36–42.
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005; 201: 1135–43.
Watanabe T, Kubota S, Nagaya M, Ozaki S, Nagafuchi H, Akashi K, et al. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res 2005; 124: 59–66.
Wang H, Li W, Goldstein R, Tracey KJ, Sama AE . HMGB1 as a potential therapeutic target. Novartis Found Symp 2007; 280: 73–85.
Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 2007; 21: 3904–16.
Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 2006; 25: 571–4.
Xu J, Beckman JS, Hogan EL, Hsu CY . Xanthine oxidase in experimental spinal cord injury. J Neurotrauma 1991; 8: 11–8.
Pineau I, Lacroix S . Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 2007; 500: 267–85.
Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN . Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine 2004; 29: 966–71.
Beattie MS, Farooqui AA, Bresnahan JC . Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma 2000; 17: 915–25.
Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS . Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur J Neurosci 2006; 24: 2567–74.
Jiang W, Bell CW, Pisetsky DS . The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol 2007; 178: 6495–503.
Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 2006; 203: 1637–42.
Sun NK, Chao CC . The cytokine activity of HMGB1 — extracellular escape of the nuclear protein. Chang Gung Med J 2005; 28: 673–82.
Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen S, et al. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology 2009; 110: 1279–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, G., Yuan, Lb., Hu, L. et al. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin 33, 11–18 (2012). https://doi.org/10.1038/aps.2011.151
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.151
Keywords
This article is cited by
-
Rodent Models of Spinal Cord Injury: From Pathology to Application
Neurochemical Research (2023)
-
Inhibition of A1 Astrocytes and Activation of A2 Astrocytes for the Treatment of Spinal Cord Injury
Neurochemical Research (2023)
-
Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury
Journal of Neuroinflammation (2020)
-
A role for spinal cord hypoxia in neurodegeneration
Cell Death & Disease (2019)
-
Inhibiting aberrant p53-PUMA feedback loop activation attenuates ischaemia reperfusion-induced neuroapoptosis and neuroinflammation in rats by downregulating caspase 3 and the NF-κB cytokine pathway
Journal of Neuroinflammation (2018)