Abstract
Aim:
To investigate the pharmacokinetic interactions induced by content variation of the main water-soluble components of Danshen injection in rats.
Methods:
Intravenous Danshen injection (control) or Danshen injection with danshensu (DSS), protocatechuic aldehyde (PAL), salvianolic acid A (Sal A) or salvianolic acid B (Sal B) were administered to female Sprague Dawley rats . Plasma concentrations of DSS, Sal A, PAL and its oxidative metabolite protocatechuic acid (PA) were analyzed simultaneously with LC-MS/MS; concentrations of Sal B were determined by the LC-MS method. Non-compartmental pharmacokinetic parameters were calculated and compared for identifying the pharmacokinetic interactions among these components.
Results:
Compared with the control group, the DSS, Sal A, and Sal B groups had significant increases in AUC0-∞ in response to elevated concentrations of PAL (by 78.1%, 51.0%, and 82.9%, respectively), while the clearances (CL) were markedly reduced (by 42.5%, 32.9%, and 46.8%, respectively). Similarly, Sal A increased the AUC0–∞ of DSS and Sal B (26.7% and 82.4%, respectively) and substantially decreased their clearances (21.4% and 45.6%, respectively). In addition, the pharmacokinetics of DSS and Sal B were significantly affected by the content variation of the other major components; the AUC0-∞ increased by 45.1% and 52.1%, respectively, the CL dropped by 29.6% and 27.1%, respectively, and the T1/2 was decreased by 22.0% and 19.6%, respectively.
Conclusion:
Complex, extensive pharmacokinetic interactions were observed among the major water-soluble constituents in the Danshen injection. The content variation of PAL had the most significant effect on the pharmacokinetic behaviors of other major constituents. Furthermore, the pharmacokinetics of DSS and Sal B were the most susceptible to the content change of other components.
Similar content being viewed by others
Introduction
In contrast to Western drugs, herbal medicines are complex products consisting of multiple compounds that achieve their multitarget therapeutic effects by the interactions between these compounds1. Recent studies have shown that pharmacological activities may differ between the isolated components and the total extracts2, 3, indicating that the interactions among these known and unknown components may play an essential role in the activity of the prescription as a whole. These differences in biological activities are also generally reflected in the pharmacokinetic behaviors of these ingredients4, 5, 6, 7. For example, baicalin in Huang-Lian-Jie-Du-Tang influenced the pharmacokinetic behavior of wogonoside5. The other ingredients in Gentiana manshurica had a remarkable influence on the pharmacokinetics of gentiopicrin6. These findings strongly suggest that more attention should be paid to effects of multicomponent pharmacokinetic interactions on the integrity of complete prescriptions.
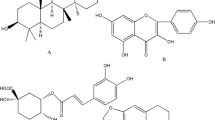
Danshen, the dried root of Salvia miltiorrhiza Bge, has been widely used in many Asian countries for the treatment of cardiovascular and cerebrovascular diseases8, 9, 10. Chemical constituent studies show that danshen primarily contains diterpenoid tanshinones and phenolic acids11, 12. Recently, the phenolic acids of danshen have gained more attention as a large pool of natural antioxidants13. In Danshen injection (DI), phenolic acids including danshensu(DSS), protocatechuic aldehyde(PAL), salvianolic acid A(Sal A), salvianolic acid B(Sal B) (Figure 1), rosmarinic acid, salvianolic acid D, and lithospermic acid14 account for more than 90% of the constituents; nearly no lipophilic constitutes have been detected15. Thus, the pharmacologic efficacy of Danshen injection should mainly relate to these phenolic acids.
However, Danshen injection has been shown to vary considerably depending on different pretreatment and manufacture processes16, 17, 18. Furthermore, the diverse geographical origins and climates of the plants cause the content of active components to differ significantly19, 20. The content variation of Sal A or Sal B is easily observed in different batches of Danshen injections, sometimes more than tenfold17, 21. This variation was also reflected in a recent fingerprint study14. Therefore, it is necessary to investigate to what extent such differences may contribute to the pharmacokinetics of these main active ingredients in Danshen injections.
In recent years there have been some reports on the pharmacokinetic interactions of danshen4, 22, 23. For example, Song et al showed the pharmacokinetics of cryptotanshinone and tanshinone IIA to be significantly affected by the oral administration of tanshinone extract in rats4. Guo et al also investigated the pharmacokinetic interactions between tanshinones and polyphenolic extracts of Salvia miltiorrhiza in rats and found the AUC of tanshinone IIA and Sal B increased remarkably after giving the mixture of tanshinones and polyphenolic extracts22. However, these studies are unable to clarify which component contributes to the pharmacokinetic interactions.
In the present study, we investigated the pharmacokinetic interactions of DSS, PAL, Sal A, and Sal B in rats using the “adding” method based on the integrity of Danshen injection. The content of each main component increased up to 10-fold of the corresponding levels in Danshen injections. Pharmacokinetic parameters were then calculated and compared. Because PAL is rapidly converted in vivo to PA, which has been shown to have obvious pharmacological activities24, 25, PA was selected as another target in our study.
Materials and methods
Chemicals and animals
Danshensu, protocatechuic aldehyde, protocatechuic acid, salvianolic acid B, and vitamin C were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Salvianolic acid A, chloramphenicol, and Danshen injection (Batch No 0903053) were kindly supplied by Chiatai Qingchunbao Pharmaceutical Co Ltd (Hangzhou, China). The DSS, PAL, Sal A, and Sal B contents were determined using a validated HPLC method to be 0.701, 0.237, 0.0121, and 0.163 g/L, respectively. Methanol, acetonitrile, and formic acid (Merck Corporation, Darmstadt, Germany) were of HPLC grade; all other reagents were of analytical grade.
Female Sprague Dawley rats (clean grade) weighing 180–220 g were obtained from the Laboratory Animal Center of Nantong University (Nantong, China, License No SCXK(Su) 2008-0010). The animals were kept on a 12 h light/dark cycle for a minimum of three days with free access to water and a standard diet before the experiments. The studies were approved by the Animal Ethics Committee of China Pharmaceutical University. Every effort was made to minimize stress to rats.
Animal studies
Rats were fasted overnight with free access to water before the experiment. Then they were randomly assigned into five groups (Group A–E, n=6 each). Group A (control group) was given Danshen injection, Groups B–E (component-adding groups) were given Danshen injection with DSS, PAL, Sal A, or Sal B, respectively. Contents of these four substances were validated using the HPLC method to ensure the quality of test samples. The administration volume of the drug solution was 10 mL/kg in all groups. After the rats were lightly anesthetized with diethyl ether, surgery was conducted to quickly expose one side femoral vein and then the drug solution was administered via the femoral vein within 30 s. Doses of DSS, PAL, Sal A, and Sal B in each group are summarized in Table 1.
Blood samples (0.2 mL) were collected from the ophthalmic venous plexus in heparinized polythene tubes immediately before drug administration and 2, 5, 10, 20, 30, 45, 60, 90, 120, and 180 min post-dosing for the simultaneous analysis of DSS, PAL, PA, and Sal A. Plasma was separated quickly using centrifugation at 1600×g for 10 min and stored at -20 °C until analysis.
For the analysis of Sal B, twenty-four rats were randomly divided into four groups (Group A'–D', n=6 each). The method of administration was the same as for the animal experiment mentioned above. Blood samples (0.2 mL) were collected before drug administration and 4, 8, 12, 16, 24, 36, 48, 64, and 80 min post-dosing. Plasma was then obtained and stored at −20 °C until analysis.
Drug assay
Determination of Danshensu, protocatechuic aldehyde, protocatechuic acid, and salvianolic acid A
The separation was performed on a Shim-pack C18 column (150 mm×2.0 mm, 5.0 μm, Shimadzu) at 35 °C with acetonitrile/water (containing 0.1% formic acid) 40:60 as a mobile phase at a flow rate of 0.2 mL/min. A TSQ Quantum Access triple-quadruple tandem mass spectrometer (Thermo Fisher Corporation, San Jose, USA) equipped with an electrospray ionization source (ESI) operated in negative ion mode was used. The mass spectrometer was operated in select reaction monitoring (SRM) mode with the spray voltage set at 3500 V and nitrogen sheath and auxiliary gas set at 40 and 5 L/min, respectively. The heated capillary temperature was set at 270 °C. The argon collision-induced dissociation (CID) was used with a gas pressure of 1.5 mTorr. Tandem mass spectrometer was tuned to monitor the m/z 197.1 → m/z 153.0 for danshensu, m/ z 137.1 → m/z 108.2 for protocatechuic aldehyde, m/z 153.0 → m/z 109.1 for protocatechuic acid, m/z 493.2 → m/ z 295.0 for salvianolic acid A, and m/z 321.0 → m/z 152.0 for chloramphenicol (IS). Injection volume was 10 μL.
The samples were prepared as follows: 100 μL plasma samples were mixed with 20 μL of vitamin C (1 g/L) and 25 μL of the IS spiking solution (chloramphenicol, 800 μg/L). Then the mixture was acidified with 100 μL of 1 mol/L HCl and vortex-mixed with 3 mL ethyl acetate for 3 min followed by centrifugation at 1600×g for 10 min. The upper organic layer was quantitatively transferred and evaporated to dryness under a gentle stream of nitrogen at 35 °C. The residue was reconstituted in 100 μL in the mobile phase; after centrifugation at 17 300×g for 10 min a 10 μL aliquot of the supernatant was injected into the LC-MS/MS system for analysis. The added component in each group was not selected in the determination due to its high concentration.
Determination of Salvianolic acid B
The quantitative analysis of salvianolic acid B was performed according to the established method with mild modifications26 using a Shimadzu 2010EV LC-ESI-MS system (Shimadzu Corporation, Tokyo, Japan) with a Shim-pack C18 column (150 mm×2.0 mm, 5.0 μm, Shimadzu). The mobile phase was methanol/water (containing 0.01% ammonium acetate) in gradient elution mode at a flow rate of 0.2 mL/min and the column temperature was maintained at 40 °C. Target ions were monitored at m/z 717.1 for salvianolic acid B and m/z 321.15 for chloramphenicol (IS) in the negative ion-monitoring mode. The plasma Sal B concentrations were determined after acetic ether liquid extraction. Briefly, a 100 μL aliquot of plasma was mixed with 25 μL chloramphenicol methanol solution (100 μg/L). Then the mixture was acidified with 40 μL HCl (2 mol/mL) and vortex-mixed with 800 μL ethyl acetate for 5 min followed by centrifugation at 17 300×g for 2 min. The upper organic layer was quantitatively transferred and evaporated to dryness using an evaporator at 40 °C. The residue was reconstituted in 100 μL of the methanol-water mixture (50:50, v/v). After centrifugation at 17 300×g for 10 min, a 10 μL aliquot of the supernatant was injected into the LC-MS system for analysis.
Pharmacokinetic analysis and statistics
Non-compartmental pharmacokinetic analysis and statistical tests
Non-compartmental pharmacokinetic analysis was performed using Excel software (Microsoft, Redmond, WA). The maximum plasma concentration (Cmax) and the time to reach the maximum concentration (Tmax) were taken directly from the observed data. The terminal elimination rate constant (ke) was estimated from the slope at the terminal phase of the log plasma concentration-time profiles and the terminal elimination half-life (T1/2) was then calculated as 0.693/ke. The area under measured plasma concentration-time curve (AUC0–t) was calculated using the linear trapezoidal method. The area under plasma concentration-time curve extrapolated to infinity (AUC0–∞) was calculated as follows: AUC0–∞=AUC0–t+Ct/ke. The mean residence time (MRT) was calculated by the moment method. Clearance (CL) and the apparent volume of distribution (Vd) were estimated by CL=Dose/AUC0–∞, Vd=CL/ke, respectively. The metabolic ratio (MR) of PAL to PA was calculated as AUC0-∞, PA/AUC0-∞, PAL.
Results were expressed as means±SD. After applying the Bartlett's test for non-homogeneity of variances, statistical analysis including the two-tailed Student's t-test or the nonparametric Mann-Whitney U test was used to evaluate the significance of the differences between the control group and the other groups. The acceptable level of significance was established at P<0.05 and P<0.01 compared with control group, respectively.
Statistical analysis of the pharmacokinetic parameter alterations of the major components
Relative to the control group, the percentage increase (%) in each pharmacokinetic parameter for each component-adding group was calculated. The contribution of the added component to the pharmacokinetic alterations of the other main ingredients was able to be ranked. One-way analysis of variance (ANOVA) was used to compare the alterations of each pharmacokinetic parameter among the component-adding groups. General linear model (GLM) univariate analysis was conducted to compare the variation of each pharmacokinetic parameter from the added component or the various analyzed ingredients. This analysis was performed using SPSS statistical software (SPSS Inc, USA).
In addition, the percentage increases of each pharmacokinetic parameter from all component-adding groups were collected to calculate the average alteration magnitude. Thus, the susceptibility of the pharmacokinetic activity for each component may be easily evaluated. For each pharmacokinetic parameter, one-way ANOVA was employed to assess the global influence of the ingredient by the other added components to Danshen injection.
Results
Pharmacokinetics of danshensu and salvianolic acid B were susceptible to the content change of other main ingredients
Pharmacokinetic parameters of danshensu and salvianolic acid B are summarized in Table 2 and their mean plasma concentration-time profiles after intravenous administration of Danshen injection with or without added components are shown in Figures 2 and 3, respectively. Compared with the control group, the clearances(CL) of DSS dropped significantly (by 42.5%, 21.4%, and 24.9% after adding PAL, Sal A, and Sal B, respectively), the Vd was markedly reduced (by 42.7%, 44.9%, and 52.4% after adding PAL, Sal A, and Sal B, respectively, P<0.01). Consequently, the AUC0–∞ of DSS was obviously elevated (by 78.1%, 26.7%, and 30.5% after adding PAL, Sal A, and Sal B, respectively). Sal A and Sal B decreased the T1/2 and mean retention time(MRT) of DSS significantly (P<0.05), indicating that the salvianolic acids influenced the terminal elimination and distribution capability of DSS.
Mean plasma concentration–time profiles of danshensu in rat plasma after intravenous administration of Danshen injection with or without added components (10 mL/kg) in rats. The dosage of DSS for each group was 7.01 mg/kg; the doses of the other ingredients are summarized in Table 1. The inset graph shows the pharmacokinetic profiles of DSS in the initial 30 min. Data are expressed as means±SD (n=6). —▴— DI (Group A); .....•..... DI+PAL (Group C); ---○--- DI+Sal A (Group D); .....×..... DI+Sal B (Group E).
Mean plasma concentration–time profiles of salvianolic acid B in rat plasma after intravenous administration of Danshen injection with or without added components (10 mL/kg) in rats. The dosage of Sal B for each group was 1.63 mg/kg and the doses of other ingredients are summarized in Table 1. The inset graph shows the pharmacokinetic profiles of Sal B in the initial 24 min. Observations are reported as means±SD (n=6). —▴— DI (Group A); ---▵--- DI+DSS (Group B); .....•.....DI+PAL (Group C); ---○--- DI+Sal A (Group D).
PAL and Sal A extensively influenced the pharmacokinetic activity of Sal B, as reflected in all of the pharmacokinetic parameters (P<0.05). They significantly increased the plasma Sal B levels. The CL was clearly reduced (by 46.8% and 45.6% after adding PAL and Sal A, respectively) and the Vd markedly decreased (by 55.7% and 62.6%, respectively). As a result, the AUC0–∞ of Sal B increased nearly 100% compared with the control group. No significant alteration in the Sal B pharmacokinetics was observed between the control group and Group B (DI+DSS).
Pharmacokinetic effects of content variation of other components on protocatechuic aldehyde and its metabolite protocatechuic acid
The mean plasma concentration-time profiles of PAL and PA after intravenous administration of Danshen injection with or without an added component in each group are shown in Figure 4, and the pharmacokinetic parameters are summarized in Table 2. Sal B was observed to significantly increase the T1/2 and Vd of PAL (by 49.7% and 63.7%, respectively); however, these two effects neutralized each other. As a result, the AUC0–∞ and CL did not change significantly. No significant differences in the AUC0–∞, MRT, and CL were found between the control and component-added groups.
Mean plasma concentration–time profiles of protocatechuic aldehyde (A) and its oxidized metabolite protocatechuic acid (B) in rat plasma after intravenous administration of Danshen injection with or without added components (10 mL/kg) in rats. The dosage of PAL was 2.37 mg/kg in all groups, and the doses of other ingredients are summarized in Table 1. The inset graph shows the pharmacokinetic profiles of PAL (A) or PA (B) in the initial 30 min. Values are reported as the means±SD (n=6). —▴— DI (Group A); ---▵--- DI+DSS (Group B); ---○--- DI+Sal A (Group D); .....×..... DI+Sal B (Group E).
By contrast, DSS had a greater impact on the pharmacokinetic behavior of the oxidative metabolite PA. The Cmax of PA markedly decreased from 1.60 mg/L to 0.88 mg/L (P<0.01) and the metabolic ratio (MR) dropped significantly (by 19.9%, P<0.05) compared with the control group, indicating that DSS significantly inhibits the conversion of PAL to PA in rats. The pharmacokinetics of PA were similar when Sal B was added compared with the control group.
Pharmacokinetic effects of content variation of other major components on salvianolic acid A
The mean plasma concentration-time profiles of Sal A after intravenous administration of Danshen injection with or without an added component are depicted in Figure 5 and the non-compartmental pharmacokinetic parameters are listed in Table 2. Compared with the control group, PAL significantly altered the pharmacokinetics of Sal A in rats. The CL and Vd markedly decreased (by 32.9% and 47.6%, respectively) and as a consequence the AUC0–∞ of Sal A increased from 23.54 to 35.59 (mg·min)/L (ie, 51.0% increase). In addition, as Table 2 indicates, the pharmacokinetic parameters of Sal A did not change significantly compared with the control group when DSS or Sal B was added.
Mean plasma concentration–time profiles of salvianolic acid A in rat plasma after intravenous administration of Danshen injection with or without added components (10 mL/kg) in rats. The dosage of Sal A was 0.121 mg/kg in all groups and the doses of other ingredients are summarized in Table 1. The inset graph shows the pharmacokinetic profiles of Sal A in the initial 30 min. Data are expressed as means±SD (n=6). —▴— DI (Group A); ---▵--- DI+DSS (Group B); .....•..... DI+PAL (Group C); .....×..... DI+Sal B (Group E).
Pharmacokinetic analysis and statistics
Detailed pharmacokinetic parameter alterations that resulted from the content changes to the Danshen injection compared with the control group are shown in Figure 6. PAL was easily observed to confer the most significant pharmacokinetic impact on the other major components in the Danshen injection. The T1/2 and Vd of DSS, Sal A, and Sal B dropped markedly (by an average 13.1% and 48.7%, respectively). Due to more significant alterations of Vd than T1/2, the clearances of danshensu and salvianolic acids decreased by a total of 40.8% when PAL was added. As a result, the AUC0-∞ of DSS, Sal A, and Sal B increased by 78.1%, 51.0%, and 82.9% respectively. Similar to PAL, Sal A had secondary effects on the pharmacokinetics of DSS, Sal B, PAL and its metabolite PA. After Sal A was added, the AUC0-∞ of these components rose by 31.4% and the CL dropped by 26.7% on average. By contrast, DSS conferred the least pharmacokinetics effects on Sal A, Sal B PAL, and PA overall. One way ANOVA analysis demonstrated the differences of the pharmacokinetic alterations to be most significant with Group D (DI+Sal A) and least significant with Group C (DI+PAL). This finding suggested that PAL has the most homogenous impact on the pharmacokinetic activity of the other components in Danshen injections. GLM univariate analysis showed the variation of the pharmacokinetic parameter values from the “added” components to be more significant than that of the various ingredients analyzed in Danshen injections. In summary, from the viewpoint of component-adding, if the average change in magnitude of overall pharmacokinetic parameters were calculated to evaluate the influences of each added component, the results could be ranked as follows: PAL>Sal A>Sal B>DSS.
Histogram plot of the percentage increases of the non-compartmental pharmacokinetic parameters compared with the control group given Danshen injection intravenously (n=6). Each bar represents one of the main analyzed ingredients in rat plasma. Subplot A–F show the percentage increases of AUC0–∞, AUC0–t, CL, MRT, T1/2, and Vd, respectively. Results are represented as means±SD.
Global pharmacokinetic parameter alterations of each major constituent in Danshen injection are summarized in Figure 7. The changes in magnitude of the pharmacokinetic parameters of DSS and Sal B were clearly the most significant, indicating that DSS and Sal B are good sensitive markers from a pharmacokinetic point of view. By contrast, the pharmacokinetic influences of PAL were mild when other major constituents were added. Furthermore, the AUC0-∞ of each parent compound (ie, DSS, PAL, Sal A, and Sal B) in the Danshen injection increased compared with the control group (30.6% elevation on average). This finding implies that a synergistic pharmacokinetic interaction occurred among these components. On the contrary, the AUC0-∞ of PA (PAL metabolite) decreased slightly (by about 1.8%). For each non-compartmental pharmacokinetic parameter the results of one way ANOVA analysis showed the differences of the parameter alterations among the major components in Danshen injection to all be significant (P<0.05). This finding indicates that the pharmacokinetic activity sensitivities of these main constituents are obviously different when other components are added. Taken together, from the determination point of view if the mean change magnitudes of the entire pharmacokinetic parameters were calculated to assess the susceptibility of each ingredient, the results may be ranked the following order: Sal B≈DSS>Sal A>PAL>PA.
Histogram representation of global pharmacokinetic parameter alterations of the main ingredients in Danshen injection. Each bar represents the average percentage increase of the non-compartmental pharmacokinetic parameter value in all component-added groups compared with the control group given Danshen injection intravenously. All data are expressed as means±SEM.
Discussion
In our experiment, a novel “adding” method was applied to investigate the pharmacokinetic interactions of the main water-soluble components of the Danshen injection. This scenario was a successful application of sensitivity analysis on the experimental design. Based on the integrity of the Danshen injection, the level of only one component varies in each component-added group while the others remain unchanged. Therefore, definite pharmacokinetic interactions may be identified. By contrast, Guo et al could not identify which component in tanshinone extracts leads to significant pharmacokinetic alteration of Sal B22. Furthermore, the magnitude and the order of the pharmacokinetic sensitivities of various components in Danshen injection could not be obtained from the results.
As shown in Figure 7, the AUC of the parent components (ie, DSS, PAL, Sal A, and Sal B) in Danshen injection increased when other components were added, while the clearance of these ingredients decreased. Thus, we conclude that synergistic pharmacokinetic interactions occurred among these parent compounds. This conclusion is consistent with previous reports7, 22. Our previous work showed that when Sal B was added the pharmacokinetics of DSS was significantly affected in plasma, tissue distribution and urine excretion27. Therefore, we presume that the pharmacokinetic interactions of these water-soluble constituents would also occur in the tissue distribution and urine excretion. The protein binding rate of salvianolic acid B has been reported to be nearly 90% in rat plasma28. Hence, PAL, and Sal A clearly influence the pharmacokinetic behavior of Sal B, possibly via the plasma protein binding replacement. In addition, the active ingredients in Salvia miltiorrhiza extract have shown inhibitory effects on the catalytic activities of cytochrome P450 enzymes29. Thus, the metabolic inhibition may be another reason for the pharmacokinetic interactions among the major components in Danshen injection. Considering that the pharmacological actions of danshen work in a dose-dependent manner30, 31, 32, the pharmacokinetic interactions within danshen water-soluble components would lead to potential pharmacodynamic interactions. Thus, more attention should be paid to the content variation of Danshen injection.
As Figure 6 indicated, PAL exerts the most significant influence on the pharmacokinetics of the other major components in Danshen injection, followed by Sal A. When PAL or Sal A was added the AUC increased by 70.7% or 31.4% on average, respectively, while the CL decreased by 40.8% or 26.7%, respectively. This finding is supported by the fact that PAL is considered a quality control component of Danshen injection33.
Our findings indicate that Sal A has a clear effect on the pharmacokinetic behavior of the other major components. The content of Sal A is low in Danshen injections and varies significantly from batch to batch16, 20, 21. In recent years, Sal A has demonstrated wide pharmacological activities such as protective effects against myocardial infarction34, diabetes35, or hepatic injury36. The change in Sal A content in Danshen injections not only induces the alteration of the pharmacological effects by itself, but also possibly leads to the alteration of the pharmacological actions of other major components. Hence, the integrated pharmacological activity of Danshen injection may be affected by the content variation of Sal A. At present, only PAL is quantified and used as a marker compound for the quality control of Danshen injection33. PAL may not reflect the real and comprehensive active constituents in Danshen injection, and is thus inadequate as a quality control. Further control of Sal A content in Danshen injections is warranted.
In summary, extensive and complex pharmacokinetic interactions were observed among the major water-soluble constituents in Danshen injections. PAL exerted the most significant effects on the pharmacokinetic behaviors of other major constituents. Meanwhile, the pharmacokinetics of DSS and Sal B were more susceptible to the content variation of other components. Further study is needed to investigate the potential pharmacodynamic changes due to the content variation of Danshen injection.
Author contribution
Xiao-quan LIU and Bo-bo CHANG designed the research; Bo-bo CHANG, Lin ZHANG, Wan-wen CAO, and Yan WANG performed the research; Bo-bo CHANG, Yuan CAO, Wen-liang YANG, and Yuan-cheng CHEN analyzed the data; Bo-bo CHANG and Yuan-cheng CHEN wrote the paper.
References
Wagner H . Multitarget therapy — the future of treatment for more than just functional dyspepsia. Phytomedicine 2006; 13: 122–9.
Ren Y, Houghton PJ, Hider RC, Howes MJ . Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorrhiza. Planta Med 2004; 70: 201–4.
Lay IS, Chin JH, Shiao MS, Lui WY, Wu CW . Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Med 2003; 69: 26–32.
Song M, Hang TJ, Zhang ZX, Chen HY . Effects of the coexisting diterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur J Pharm Sci 2007; 32: 247–53.
Lu T, Song J, Huang F, Deng YX, Xie L, Wang GJ, et al. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix Scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol 2007; 110: 412–8.
Jiang SH, Kang LJ, Ye J, Yao SK, Liu WN . Studies on pharmacokinetics of gentiopicrin in longdan and its compound preparation in rats. Chin Pharm J 2005; 40: 212–5. Chinese.
Song M, Hang TJ, Zhang ZX . Pharmacokinetic interactions between the main components in the extracts of Salvia miltiorrhiza Bge in rat. Yao Xue Xue Bao 2007; 42: 301–7. Chinese.
Cheng TO . Cardiovascular effects of Danshen. Int J Cardiol 2007; 121: 9–22.
Wu B, Liu M, Liu H, Li W, Tan S, Zhang S, et al. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 2007; 38: 1973–9.
Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther 2008; 117: 280–95.
Wang X, Morris-Natschke SL, Lee KH . New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev 2007; 27: 133–48.
Zhou LM, Zuo Z, Chow MS . Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005; 45: 1345–59.
Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman M, Zhu YC . Antioxidants in Chinese herbal medicines: a biochemical perspective. Nat Prod Rep 2004; 21: 478–89.
Zhang JL, Cui M, He Y, Yu HL, Guo DA . Chemical fingerprint and metabolic fingerprint analysis of Danshen injection by HPLC-UV and HPLC-MS methods. J Pharm Biomed Anal 2005; 36: 1029–35.
Liu AH, Lin YH, Yang M, Guo H, Guan SH, Sun JH, et al. Development of the fingerprints for the quality of the roots of Salvia miltiorrhiza and its related preparations by HPLC DAD and LC-MS(n). J Chromatogr B Analyt Technol Biomed Life Sci 2007; 846: 32–41.
Chang YX, Ding XP, Qi J, Cao J, Kang LY, Zhu DN, et al. Determination of phenolic acids in danshen preparations by LC with chemiluminescence detection. Chromatographia 2009; 69: 319–23.
Xu JZ, Shen J, Cheng YY, Qu HB . Simultaneous detection of seven phenolic acids in Danshen injection using HPLC with ultraviolet detector. J Zhejiang Univ Sci B 2008; 9: 728–33.
Liu AH, Li L, Xu M, Lin YH, Guo HZ, Guo DA . Simultaneous quantification of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLC-DAD method. J Pharm Biomed Anal 2006; 41: 48–56.
Li MH, Chen JM, Peng Y, Wu QL, Xiao PG . Investigation of danshen and related medicinal plants in China. J Ethnopharmacol 2008; 120: 419–26.
Zhong GX, Li P, Zeng LJ, Guan J, Li DQ, Li SP . Chemical characteristics of Salvia Miltiorrhiza (danshen) collected from different locations in China. J Agric Food Chem 2009; 57: 6879–87.
Yu Dan . The effects of other components on the pharmacokinetics of danshensu in Danshen injection [dissertation]. Nanjing: China Pharm Univ; 2009.
Guo ZJ, Zhang Y, Tang X, Li H, Sun QS . Pharmacokinetic interaction between tanshinones and polyphenolic extracts of Salvia miltinorrhiza BUNGE after intravenous administration in rats. Biol Pharm Bull 2008; 31: 1469–74.
Lin L, Liu JX, Zhang Y, Lin CR, Li LQ . Effect of different combinations of compound Danshen preparation on pharmacokinetic parameters of salvianolic acid B in rat plasma. China J Chin Mater Med 2008; 33: 2683–7. Chinese.
Cao YG, Zhang L, Ma C, Chang BB, Chen YC, Tang YQ, et al. Metabolism of protocatechuic acid influences fatty acid oxidation in rat heart: new anti-angina mechanism implication. Biochem Pharmacol 2009; 77: 1096–104.
Guan S, Ge D, Liu TQ, Ma XH, Cui ZF . Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol In Vitro 2009; 23: 201–8.
Shao JP, Zhang SF, Chen YC, Yang WL, Liu XQ . Cellular uptake characteristics of salvianolic acid B in myocardial cells and blood vessel endothelial cells. J China Pharm Univ 2009; 263–8. Chinese.
Yu D, Chai JG, Cao YG, Chen YC, Liu XQ . Effects of salvianolic acid B on the pharmacokinetics of danshensu in Danshen injection in rats. J China Pharm Univ 2009; 40: 258–62. Chinese.
Ma L, Ren WC, Dong J, He H, Chen XJ, Wang GJ . Pharmacokinetics of salvianolic acid B after intravenous administration in rats. Chin J Clin Pharmacol Ther 2007; 12: 1231–6.
Qiu FR, Zhang R, Sun JG, A JY, Hao HP, Peng Y, et al. Inhibitory effects of seven components of danshen extract on catalytic activity of cytochrome P450 enzymes in human liver microsomes. Drug Metab Dispos 2008; 36: 1308–14.
Kang DG, Yun YG, Ryoo JH, Ryoo JH, Lee HS . Anti-hypertensive effect of water extract of danshen on renovascular hypertension through inhibition of the renin angiotensin system. Am J Chin Med 2002; 30: 87–93.
Kim JY, Kim HS, Kang HS, Choi JS, Yokozawa T, Chung HY . Antioxidant potential of dimethyl lithospermate isolated from Salvia miltiorrhiza (Red Sage) against peroxynitrite. J Med Food 2008; 11: 21–8.
Nakazawa T, Xui N, Hesong Z, Kinoshita M, Chiba T, Kaneko E, et al. Danshen inhibits oxysterol-induced endothelial cell apoptosis in vivo. J Atheroscler Thromb 2005; 12: 132–7.
The Pharmacopoeia Commission of Ministry of Health of PRC (Eds), Drug standards, Beijing: The Pharmacopoeia Commission of Ministry of Health of PRC; 1998.
Wang SB, Tian S, Yang F, Yang HY, Yang XY, Du GH . Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol 2009; 615: 125–32.
Wang SB, Yang XY, Tian S, Yang HG, Du GH . Effect of salvianolic acid A on vascular reactivity of streptozotocin-induced diabetic rats. Life Sci 2009; 85: 499–504.
Hu YY, Liu CH, Wang RP, Liu C, Liu P, Zhu DY . Protective actions of salvianolic acid A on hepatocyte injured by peroxidation in vitro. World J Gastroenterol 2000; 6: 402–4.
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No 30772609). The authors thank Chiatai Qingchunbao Pharmaceutical Co Ltd (Hangzhou, China) for providing Danshen injection and standard substances. The authors also appreciate Ms Hua HE, Yu CHENG, Li-hua HUANG, and Qian SI for their technical help with the animal study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, Bb., Zhang, L., Cao, Ww. et al. Pharmacokinetic interactions induced by content variation of major water-soluble components of Danshen preparation in rats. Acta Pharmacol Sin 31, 638–646 (2010). https://doi.org/10.1038/aps.2010.27
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.27
Keywords
This article is cited by
-
HPLC and LC-MS analysis of sinomenine and its application in pharmacokinetic studies in rats
Acta Pharmacologica Sinica (2010)