Abstract
Aim:
To investigate the effect of balsalazine treatment on intestinal mucosal permeability in dextran sulfate sodium (DSS)-induced colitis and to determine the mechanism of the balsalazine-induced changes.
Methods:
Experimental colitis was induced in C57BL/6J mice by the administration of 5% DSS. Balsalazine was administered intragastrically at doses of 42, 141, and 423 mg/kg. The disease activity index (DAI) score was evaluated and colon tissue was collected for the assessment of histological changes. The amount of malondialdehyde (MDA) in the colon was determined, along with the activity of myeloperoxidase (MPO), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). Mucosa from the small intestine was collected to determine the levels of tumor necrosis factor (TNF)-α and interferon (IFN)-γ. The mucosa was ultrastructurally examined with transmission electron microscopy and intestinal permeability was assayed using Evans blue.
Results:
Balsalazine was found to reduce the DAI score and the histological index (HI) score, decrease the MDA content and the activity of MPO, and increase the activity of SOD and GSH-Px in colitis mice. At the same time, balsalazine ameliorated microvillus and tight junction structure, resulting in a decrease in the amount of Evans blue permeating into the intestinal wall and the levels of TNF-α and IFN-γ in colitis mice.
Conclusion:
In colitis mice, the anti-colitis effect of balsalazine results in a decrease in intestinal mucosal permeability. The mechanism of this effect is partly associated with balsalazine's antioxidative and anti-inflammatory effects.
Similar content being viewed by others
Introduction
The intestinal mucosal barrier plays a pivotal role in preventing microorganisms and bacterial toxins from entering an organism's bloodstream. However, the barrier becomes impaired during inflammatory bowel disease (IBD). As a result, a large quantity of endotoxin can enter into systemic circulation through the impaired intestinal mucosa1, 2. In addition, oxygen free radicals (OFRs) and proinflammatory cytokines are induced, facilitating impairment of intestinal mucosal permeability3, 4, 5, 6, 7, 8, 9. Oxidants alter cytoskeletal components (such as actin), resulting in disruption of the structural integrity of epithelial cells10, 11. In addition, proinflammatory cytokines, including tumor necrosis factor (TNF)-α and interferon (IFN)-γ, disrupt tight junctions6, 7, 8, 9, decrease the transendothelial electrical resistance (TEER), and regulate the expression of intestinal mucosal barrier-associated proteins. Therefore, restoring the impaired intestinal mucosa is beneficial for controlling and reducing the inflammation and immunologic reaction occurring in the intestinal mucosa of patients with IBD12.
5-aminosalicylate (5-ASA) is used to treat ulcerative colitis (UC) because of its ability to control and relieve inflammation. Sulfasalazine, the first 5-ASA-containing drug, functions by releasing an active component in the colon through the activity of azo reductase expressed by colonic bacterial. Sulfasalazine has been approved for therapeutic usage because of its ability to improve intestinal mucosal permeability13, 14. Balsalazine (5-ASA azo bonded to an inert carrier, 4-amino-benzoyl-alanine) can also release 5-ASA through cleavage of the compound by azo reductase expressed by intestinal luminal bacteria. The administration of balsalazine effectively induces remission in patients with mild to moderate UC. However, whether balsalazine improves intestinal mucosal permeability is still unknown15, 16.
Previous studies have shown that mucosal barrier dysfunction is a pathophysiological feature of colitis, irrespective of etiology or species17, 18. Therefore, dextran sulfate sodium (DSS)-induced colitis is considered an appropriate model of the injured mucosal barrier19. The epithelium of the small intestine is at the front line of the intestinal barrier. Thus, increased small-intestine permeability may be an important etiological event in colitis20. Therefore, the present study was designed to investigate the effect of balsalazine on small-intestine mucosal permeability in the DSS-induced colitis model and the possible mechanisms of its effects.
Materials and methods
Animals and reagents
Specific pathogen-free (SPF)-grade C57BL/6J mice, 6–8 weeks old and weighing 20±2 g, were provided by Shanghai Slac Laboratory Animal Co Ltd (Shanghai, China; Certificate No SCXK 2007-0005). The mice were housed in animal facilities with 50% humidity and a 12:12-h light-dark cycle and were fed a standard pellet diet and tap water ad libitum. Balsalazine was provided by Shanxi Anter Incorporated. DSS (molecular weight 8000) was purchased from Sigma-Aldrich Co. Kits for detecting Myeloperoxidase (MPO; batch number: 080416), glutathione peroxidase (GSH-Px; batch number: 080320), malondialdehyde (MDA; batch number: 080321), superoxide dismutase (SOD; batch number: 080320) and Evans blue (EB) were all bought from Nanjing Jiancheng Biotechnology Institute (Nanjing, China). The ELISA kits for TNF-α (batch number: 080621) and IFN-γ (batch number: 080510) were obtained from Jingmei Biotech Co Ltd (Shenzhen, China). The main instruments used in this study were an ultraviolet spectrophotometer (752N; Shanghai, China), an enzyme-labeling instrument (ELx800; USA), a transmission electron microscope (TEM; Hitachi, Japan), a light microscope (Olympus; Japan), and a high-speed controllable homogenizer (FSH 2; China).
Experimental protocols
All experiments were performed in accordance with the institutional and national guidelines for the care and use of laboratory animals and were approved by the Ethics Committee of Anhui Medical University. Forty-five C57BL/6J mice were randomly divided into the following five groups: a normal group, a DSS-treated group, and three balsalazine groups treated at doses of 42 mg/kg, 141 mg/kg, and 423 mg/kg according to the study of Kimura et al. Balsalazine was resuspended in distilled water and administered intragastrically to the treatment groups once a day from the first day (d1) of the experiment and lasting for 7 days. All the other groups received distilled water as a control21.
Induction of colitis
Acute colitis was induced in mice by replacing normal drinking water with distilled water containing 5% (w/v) DSS and allowing them to drink freely for 7 days22. The mice in the DSS group and the three balsalazine groups all drank 5% DSS, and the mice in the control group received regular distilled water.
Evaluation of DAI
Throughout the duration of the experiment, the following parameters were recorded for each mouse daily by two unblinded observers: weight, presence of occult or gross blood in feces, and stool consistency. These parameters were each assigned a score and utilized to calculate an average daily disease activity index (DAI) score for each mouse as previously described22.
Surgical procedure
The mice were sacrificed by chloral hydrate anesthesia after 7 days of DSS administration. The abdomens were opened along the median line, and the colon was rapidly excised, rinsed gently with ice-cold phosphate-buffered saline, placed on ice, and opened longitudinally. Two continuous pieces were collected after gross morphological changes of colon mucosa were examined. One part of the colon was immediately fixed in 10% neutral buffered formalin for histological analysis. The other colon segment was homogenized for use in the assessment of MDA content, along with the activity of MPO, SOD, and GSH-Px. The homogenate was stored at -20 ºC. Small-intestine mucosa was collected for TEM analysis, assessment of permeability by Evans blue and levels of TNF-α and IFN-γ.
Histological assessment
The portion of the colon fixed in 10% neutral buffered formalin was embedded in paraffin for histological analysis. Full-thickness (5 μm) sections were stained with hematoxylin and eosin and examined microscopically by a blinded pathologist who was unaware of the experimental design of the study. Severity of colitis was graded on a scale of 0–4 and expressed as the pathological index according to the standard scoring system22.
Assessment of TEM
The ileum within 0.5 cm of the ileocecal junction (about 1 cm) was excised with a sharp scalpel. Specimens processed for TEM were fixed in 2.5% glutaraldehyde for four hours at 4 ºC, followed by fixation in osmic acid and embedded in Epon. Ultrathin sections were examined by TEM.
Determination of intestinal permeability by Evans blue
The small-intestine sacs were prepared as previously described23, 24. Briefly, the small intestine was incised, and the fecal contents were washed out gently with 2−3 mL of PBS. The proximal and distal intestines were ligated, and 0.2 mL of 1.5% (w/v) Evans blue (EB) in PBS was infused into the lumen. The sac was then incubated in 20 mL Krebs buffer in 95% O2 at 37 ºC for 30 min. The sac was washed three times in 6 mmol/L acetylcysteine and dried on filter paper at 37 ºC for 24 h, followed by incubation with 1 mL of formamide at 50 ºC for 24 h. The amount of dye eluted was estimated at a wavelength of 655 nm. The amount of EB permeating the intestinal wall was calculated based on the standard curve of EB in formamide.
Detections of colonic and intestinal homogenates
The dissected colon and intestine were excised and all fat and mesenteries were removed. The tissue was subsequently homogenized in physiologic saline and stored at -20 ºC temporarily. Detection of the amount of MDA, and the activities of MPO, SOD, and GSH-PX were determined according to the manufacturer's guidelines. The levels of TNF-α and IFN-γ in small intestine homogenates were determined using ELISA kits.
Statistical analysis
The statistical software used was SPSS11.5. All analyses are expressed as mean±standard deviation (SD). Group comparisons were performed using the one-way analysis of variance (ANOVA) test and correlations were tested by Pearson's rank correlation coefficient. A P value <0.05 was considered statistically significant.
Results
The effect of balsalazine on DSS-induced colitis
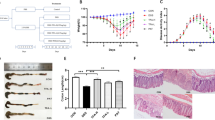
Administration of a 5% DSS solution in the drinking water resulted in colitis in these mice. The induction of colitis is reflected by the presence of bloody stool, diarrhea and weight loss. The DAI score increased from day 5 (d 5) and all mice had abnormal stools during the experiment. Balsalazine treatment significantly decreased the DAI score compared with the DSS group (P<0.05, Figure 1).
Effects of balsalazine on DAI score with administration of 5% DSS during 7 days (n=9). Animal weights, presence of occult or gross blood in the feces, and stool consistency were recorded daily for each animal to calculate an averaged daily disease activity index (DAI). (A) Normal group; (B) DSS group; (C1) balsalazine 42 mg/kg; (C2) balsalazine 141 mg/kg; (C3) balsalazine 423 mg/kg. DAI was significantly high and increasing daily in the DSS group. DAI in the balsalazine groups were lower than that in the DSS group (P<0.05 from d 5 by ANOVA test).
Colonic mucosal lesions were evaluated at the end of the experiment. Balsalazine attenuated mucosal hyperemia and edema. Additionally, balsalazine reduced the extent of colonic mucosal lesions by reducing the HI score from 7.66±1.00 in the DSS group to 5.66±0.71, 4.11±0.78, and 2.22±0.44 in the 42 mg/kg, 141 mg/kg, and 423 mg/kg balsalazine dosage groups, respectively (Figure 2). As shown in Figure 3B, DSS treatment induced multifocal superficial ulcers in the colon mucosa, inflammatory cells infiltration mainly into the mucosa and the submucosa, along with edema in the submucosa and the folliculus lymphaticus. Furthermore, surface epithelial cells and colonic crypts were damaged and severely lost. However, in the balsalazine treatment groups there was mild inflammation infiltration, integrated surface epithelium, and attenuated crypt cell loss (Figure 3C). These results indicated that balsalazine had significant anti-colitis effects.
Effects of balsalazine on HI score with administration of 5% DSS after 7 days (n=9). (A) Normal group; (B) DSS group; (C1) balsalazine 42 mg/kg; (C2) balsalzine 141 mg/kg; (C3) balsalazine 423 mg/kg. Severity of colitis was graded on a scale of 0–4 and expressed as the pathological index according to the standard scoring system. HI was significantly high in the DSS group. HI in the balsalazine groups was lower than that in the DSS group. (bP<0.05, cP<0.01 vs DSS group by ANOVA test).
Histopathologic features of the colon in association with colitis (HE×400). A: normal group, B: DSS group, C: balsalazine group. No histological damage was seen in the normal group. The DSS group showed severe inflammatory cells infiltration in mucosa and submucosa, and the surface epithelial cell and crypts were damaged and lost severely. The balsalazine group showed mild inflammation, integrated surface epithelium, and attenuated crypt cell loss.
The effect of balsalazine on intestinal mucosal barrier function in DSS-induced colitis
The integrity of the intestinal mucosal barrier is the result of the structure of the intestinal epithelial cells and the intercellular tight junctions. Compared with the control group (Figure 4A), the DSS group (Figure 4B) displayed the following changes: a focal reduction of intestinal microvillus, disarrangement of the epithelial surface, irregular widening of the intercellular space, decurtated and broader junctional complexes, and partial surface epithelium injury, even detachment of the epithelium. In contrast, the balsalazine-treated groups (Figure 4C, 4D, and 4E) displayed an attenuation of the surface epithelium injury, regular and intensive microvilli, and ameliorated tight junctions.
Effects of balsalazine on ileal mucosal epithelial structure under TEM (×20 000). A: normal group, B: DSS group, C, D, E: balsalazine 42, 141, and 423 mg/kg, respectively. No obvious microstructural damage was seen in the normal group. In the DSS group, reduced and shorter microvillus, irregular widening of the intercellular space, decurtated and broaden junctional complexes were seen. The balsalazine groups showed a much more regular and intensive microvillus, and ameliorated tight junctions dose-dependently.
Simultaneously, the amount of EB permeating into the intestinal wall in the DSS-induced group was 281.43±7.06 μg/g, which was markedly higher than that in the control group (90.07±5.20 μg/g, P<0.01). These data indicate that intestinal mucosal permeability was increased in DSS-induced colitis mice. The treatment of DSS-induced colitis mice with 42 mg/kg, 141 mg/kg, and 423 mg/kg of balsalazine lowered the amount of EB permeating into the intestinal wall to 210.99±6.55 μg/g, 166.30±7.14 μg/g, and 110.47±6.78 μg/g, respectively (P<0.05). These results indicate that balsalazine treatment protects the intestinal mucosal barrier in this colitis model.
The effect of balsalazine on intestinal mucosal oxidative damage in the DSS-induced colitis model
Compared with the control group, administration of DSS increased the amount of MDA and the activity of MPO in the colon, while the activity of both SOD and GSH-Px was decreased (P<0.01). All three dosages of balsalazine reversed the DSS-induced changes described above (P<0.05, Table 1). The correlation coefficients between DAI and either MDA, SOD or GSH-Px were 0.718, -0.837, and -0.839, respectively. The correlation coefficients between HI and either MDA, SOD, or GSH-Px were 0.818, -0.946, and -0.957, respectively. The correlation coefficients between the amount of EB and either MDA, SOD, or GSH-Px were 0.842, -0.960, and -0.900, respectively. These results indicated that balsalazine could scavenge free radicals and attenuate the oxidative damage. In addition, there was a strong correlation between oxidative indexes and the amount of EB.
The effect of Balsalazine on intestinal TNF-α and IFN-γ levels in DSS-induced colitis
Compared with the control group, DSS administration significantly increased the levels of intestinal TNF-α and IFN-γ (P<0.01). These data indicate significant intestinal inflammation in the DSS group. However, the levels of intestinal TNF-α and IFN-γ were much lower in the balsalazine treated groups than that in the DSS group (P<0.05, Table 2). The correlation coefficients between the amount of EB and the levels of intestinal TNF-α or IFN-γ were 0.887 and 0.948, respectively. These results indicate that balsalazine reduces the level of proinflammatory cytokines and attenuates the intestinal inflammation.
Discussion
In the present study, the treatment of colitis mice with balsalazine resulted in an obvious anti-colitis effect. Physiologically, balsalazine attenuated or prevented bloody stool, diarrhea, and weight loss. In addition, the administration of balsalazine decreased both the DAI and the HI score and lowered MPO activity in the DSS-induced model of colitis. The effect of balsalazine on intestinal mucosal permeability is usually reflected by changes in intestinal function and structure. An increase in the amount of EB permeating into the intestinal wall, a disruption in the epithelial cell borders, and damage to the intestinal epithelial cells and intercellular tight junctions all indicated that intestinal mucosal permeability was increased in the DSS group19, 23. Administration of balsalazine significantly decreased the DSS-induced increase in intestinal mucosal permeability. Therefore, these results indicate that the beneficial effects of balsalazine on the intestinal mucosal barrier may lead to depression of the intestinal inflammatory pathology and subsequent attenuation of colonic inflammation. However, the underlying mechanisms of the intestinal mucosal permeability improved by balsalazine treatment have not been studied. Understanding these mechanisms will contribute to elucidating the roles of balsalazine in the pathogenesis of UC.
Oxygen free radicals (OFR) play an important role in the pathogenesis of UC25 and have been shown to damage the intestinal mucosal barrier26. The loss of antioxidant defenses may severely compromise the inflamed mucosa and render it more susceptible to the oxygen-induced injury and thereby hinder the recovery of the mucosa and the return of the integrity of the epithelial cell layer. Thus, it increased the intestinal mucosal permeability27, 28, while antioxidants treatment is beneficial to ameliorate intestinal mucosa permeability29, 30, 31, 32, 33, 34. In the present study, balsalazine attenuated intestinal oxidative damage markedly by decreasing MDA content, along with increasing SOD and GSH-Px activity. Thus, considering the strong correlation between oxidative indexes and EB, we presume that the anti-oxidation effect of balsalazine might contribute to the improvement of the intestinal mucosal permeability.
IFN-γ and TNF-α are considered the most important cytokines in the pathogenesis of UC. The interaction between IFN-γ, TNF-α, and the mucosal immune system may lead to the disruption of the epithelial barrier. TNF-α can also stimulate the synthesis of OFR and other inflammatory mediators and thus amplify the disruption of epithelial barrier35. Furthermore, apoptosis of ileal enterocytes can be prevented by both anti-TNF strategies and in TNFR-1–/– animals36, 37. In our study, balsalazine decreased the levels of intestinal IFN-γ and TNF-α. These results are similar to the results described by Fiorucci et al38. Furthermore, Kim et al showed that 5-ASA attenuated TNF-dependent NF-κB activation, which may be critical during intestinal inflammation39. Considering these results, and the strong correlation between the intestinal levels of IFN-γ and TNF-α and the amount of EB, we hypothesize that balsalazine protects the intestinal mucosal barrier partly by inhibiting the proinflammatory cytokines.
In conclusion, the beneficial anti-colitis effect of balsalazine treatment was due to its ability to restore both the structure and function of the intestinal mucosal barrier. The mechanisms by which balsalazine restored the integrity of the intestinal mucosal barrier are partially associated with the antioxidative and anti-inflammatory effect of balsalazine. However, these mechanisms require further investigation. More advanced methods, including molecular biological techniques for detecting changes in the intestinal mucosal barrier-associated proteins, will be applied in future studies.
Author contributions
Xiao-chang LIU designed and carried out the study. Qiao MEI and Jian-ming XU designed the study. Jing HU carried out part of study together with Xiao-chang LIU.
References
Gitter AH, Wullstein F, Fromm M, Schulzke JD . Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 2001; 121: 1320–8.
Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999; 116: 301–9.
Simmonds NJ, Allen RE, Stevens TR, van Someren RN, Blake DR, Rampton DS . Chemiluminescense assay of mucosal reactive oxgen metabolites in inflammatory bowel disease. Gastroenterology 1992; 103: 186–96.
Kitahara T, Suzuki K, Asakura H, Yoshida T, Suematsu M, Watanabe M, et al. Active oxygen species generated by monocytes and polymorphonuclear cells in Crohn's disease. Dig Dis Sci 1988; 33: 951–5.
Babbs CF . Oxygen radicals in ulcerative colitis. Free Radic Biol Med 1992; 1: 169–81.
Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL . Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut 2003; 52: 224–30.
Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006; 131: 1153–63.
Willemsen LE, Hoetjes JP, van Deventer SJ, van Tol EA . Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol 2005; 142: 275–84.
Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG . Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 2002; 13: 3218–34.
Banan A, Zhang Y, Losurdo J, Keshavarzian A . Carbonylation and disassembly of the F-actin cytoskeleton in oxidant induced barrier dysfunction and its prevention by epidermal growth factor and transforming growth factor alpha in a human colonic cell line. Gut 2000; 46: 830–7.
McKenzie SJ, Baker MS, Buffinton GD, Doe WF . Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest 1996; 98: 136–41.
Mankertz J, Schulzke JD . Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 2007; 23: 379–83.
Wang FJ, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR . Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005; 166: 409–19.
Crotty B, Hoang P, Dalton HR, Jewell DP . Salicylates used in inflammatory bowel disease and colchicine impair interferon-gamma induced HLA-DR expression. Gut 1992; 33: 59–64.
Pruitt R, Hanson J, Safdi M, Wruble L, Hardi R, Johanson J, et al. Balsalazine is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am J Gastroenterol 2002; 7: 3078–86.
Mackowiak JI . A two-stage decision analysis to assess the cost of 5-aminosalicylic acid failure and the economics of balsalazine versus mesalamine in the treatment of ulcerative colitis. Manag Care Interface 2006; 19: 39–46, 56.
Di Paolo MC, Merrett MN, Crotty B, Jewell DP . 5-Aminosalicylic acid inhibits the impaired epithelial barrier function induced by gamma interferon. Gut 1996; 38: 115–9.
Stein J, Ries J, Barrett KE . Disruption of intestinal barrier function associated with experimental colitis: possible role of mast cells. Am J Physiol 1998; 274: 203–9.
Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2007; 2: e1308.
Arrieta MC, Madsen K, Doyle J, Meddings JB . Reducing small intestinal permeability attenuates colitis in the IL-10 gene deficient mouse. Gut 2009; 58: 41–8.
Kimura I, Kawasaki M, Nagahama S, Matsuda A, Kataoka M, Kokuba Y . Determination of the active moiety of BX661A, a new therapeutic agent for ulcerative colitis, by studying its therapeutic effects on ulcerative colitis induced by dextran sulfate sodium in rats. Arzneimittelforschung 1998; 48: 1091–6.
Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR . Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003; 52: 713–9.
Kitajima S, Takuma S, Morimoto M . Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim 1999; 48: 137–43.
Berruet N, Sentenac S, Auchere D, Gimenez F, Farinotti R, Fernandez C . Effect of efavirenz on intestinal p-glycoprotein and hepatic p450 function in rats. J Pharm Pharm Sci 2005; 8: 226–34.
Pavlick KP, Laroux FS, Bojarski C . Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 2002; 33: 311–22.
Park JH, Ma L, Oshima T, Carter P, Coe L, Ma JW, et al. Polynitroxylated starch/TPL attenuates cachexia and increased epithelial permeability associated with TNBS colitis. Inflammation 2002; 26: 1–11.
Buffinton GD, Doe WF . Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med 1995; 19: 911–8.
Mulder TP, Verspaget HW, Janssens AR . Decrease in two intestinal copper/zinc containing proteins with antioxidant function in inflammatory bowel disease. Gut 1991; 32: 1146–50.
Suzuki Y, Matsumoto T, Okamoto S, Hibi T . A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis 2008; 21.
Giriş M, Depboylu B, Doğru-Abbasoğlu S, Erbil Y, Olgac V, Alis H, et al. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin Exp Immunol 2008; 152: 102–10.
Mckenzie SJ, Baker MS, Buffinton GD, Doe WF . Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest 1996; 98: 136–41.
Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M . Role of reactive oxygen metabolites in experimemtal colitis. Gut 1990; 31: 786–90.
Grisham MB . Oxidants and free radicals in inflammatory bowel disease. Lancet 1994; 344: 859–61.
Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van Swam II, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis 2006; 12: 1044–52.
Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH, et al. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immumol 1997; 27: 1743–50.
Shen C, de Hertogh G, Bullens DM, Van Assche G, Geboes K, Rutgeerts P, et al. Remission-inducing effect of anti-TNF monoclonal antibody in TNBS colitis: mechanisms beyond neutralization? Inflamm Bowel Dis 2007; 13: 308–16.
Fries W, Muja C, Crisafulli C, Costantino G, Longo G, Cuzzocrea S, et al. Infliximab and etanercept are equally effective in reducing enterocyte apoptosis in experimental colitis. Int J Med Sci 2008; 5: 169–80.
Fiorucci S, Distrutti E, Ajuebor MN, Mencarelli A, Mannucci R, Palazzetti B, et al. No-mesalamine protects colonic epithelial cells against apoptotic damage induced by proinflammatory cytokines. Am J Physiol Gastrointest Liver Physiol 2001; 281: 654–65.
Kim H, Jeon H, Kong H, Yang Y, Choi B, Kim YM, et al. A molecular mechanism for the anti-inflammatory effect of taurine-conjugated 5-aminosalicylic acid in inflamed colon. Mol Pharmacol 2006; 69: 1405–12.
Acknowledgements
We thank Ke CHEN and Wen HU for assistance with pathological technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Xc., Mei, Q., Xu, Jm. et al. Balsalazine decreases intestinal mucosal permeability of dextran sulfate sodium-induced colitis in mice. Acta Pharmacol Sin 30, 987–993 (2009). https://doi.org/10.1038/aps.2009.77
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.77
Keywords
This article is cited by
-
Gut microbiota of old mice worsens neurological outcome after brain ischemia via increased valeric acid and IL-17 in the blood
Microbiome (2023)
-
RETRACTED ARTICLE: Ameliorative effect of two structurally divergent hydrazide derivatives against DSS-induced colitis by targeting Nrf2 and NF-κB signaling in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)
-
SLC26A3 (DRA) prevents TNF-alpha-induced barrier dysfunction and dextran sulfate sodium-induced acute colitis
Laboratory Investigation (2018)
-
Co-supplementation of isomalto-oligosaccharides potentiates metabolic health benefits of polyphenol-rich cranberry extract in high fat diet-fed mice via enhanced gut butyrate production
European Journal of Nutrition (2018)
-
Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella)
Scientific Reports (2017)