Abstract
Aim:
This study was conducted to demonstrate the anti-atherosclerotic effect of dehydroepiandrosterone (DHEA) and to investigate its possible mechanisms and whether this effect is related to its conversion to estrogen.
Methods:
Forty male New Zealand White rabbits aged 3 months were divided into 5 groups (n=8 per group) and fed different diets for 10 weeks. Serum lipid levels, the area of atherosclerotic lesions and the mRNA levels of monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) in aortic lesions were measured. Then cultured vascular smooth muscle cells (VSMCs) stimulated by oxidized low density lipoprotein-cholesterol (ox-LDL) were treated by DHEA. The gene and protein expression levels of MCP-1 and VCAM-1 in VSMCs was detected. The plasmid with or without the gene of cytochrome P450 aromatase (CYP19) was transient transfected into cultured VSMCs respectively. Twenty hours later, the cells were stimulated with ox-LDL and DHEA.
Results:
DHEA could obviously decrease the area of atherosclerotic lesions and the expressions of MCP-1 and VCAM-1 in aortic lesions. But all-trans retinoic acid (atRA) which was reported would limit restenosis after balloon angioplasty had no visible synergistic effect with DHEA. DHEA could also reduce ox-LDL-induced MCP-1 and VCAM-1 expression in untransfected or transfected VSMCs.
Conclusion:
The anti-atherosclerotic effect of DHEA had nothing to do with the catalysis of cytochrome P450 aromatase (CYP19), or was not related to its conversion to estrogen.
Similar content being viewed by others
Introduction
Dehydroepiandrosterone (DHEA) is a C-19 steroid that is synthesized mainly by the human adrenal cortex. Its plasma levels significantly increase following puberty, but decline with advancing age1. Therefore, some scholars refer to DHEA as the youth hormone2. DHEA and its sulfated ester, DHEA sulfate (DHEAS), are produced in higher levels than any other circulating steroid hormone. Most circulating DHEA is in the form of DHEAS, which functions as an inactive reservoir for DHEA. Epidemiological observations and animal experiments indicate that DHEA has a wide variety of beneficial biological and physiological effects, including prevention of atherosclerosis3, 4, 5, 6, 7. However, the mechanisms responsible for the anti-atherosclerosis effects of DHEA are largely unknown. Several investigations have proposed that DHEA is converted enzymatically to testosterone and estradiol in peripheral tissues to play roles8, 9.
One of the earliest detectable cellular responses in the formation of atherosclerotic lesions is increased adhesion of mononuclear cells to the injured endothelium, followed by their extravasation into the vessel wall10. Exactly how these cells are recruited and retained in the artery wall remains unclear, but researchers speculate that cell adhesion/chemoattractant molecules play a key role in this process11, 12. Various adhesion molecules have been identified in atherosclerosis, including vascular adhesion molecule-1 (VCAM-1, CD106). The expression of VCAM-1 mediates the binding of monocytes and lymphocytes to vascular endothelial cells through interactions with a counterreceptor, the very late activation antigen-1 (VLA-1)13. In hypercholesterolemic animals, VCAM-1 is upregulated over early foam cell lesions, particularly at the periphery14, where monocyte adhesion is maximal15. Monocyte chemoattractant protein (MCP)-1 which belongs to the CC subfamily of the chemokine family, is an important chemokine that stimulates the migration of monocytes into the intima of the arterial wall12. It has been demonstrated that MCP-1 expression occurs in the arterial wall in response to hypercholesterolemia in rabbits. Oxidized LDL also induces local vascular cells to produce monocyte chemotactic protein-1 (MCP-1), which causes monocyte recruitment and promotes the release of lipids and lysosomal enzymes into the extracellular space, thereby enhancing the progression of the atherosclerotic lesion16. Various types of cells such as vascular smooth muscle cells are known to produce MCP-1 in response to diverse stimuli, including proinflammatory cytokines and pathological microorganisms17. The expression levels of MCP-1 and VCAM-1 may provide corresponding severity levels of atherosclerosis.
Accordingly, this study was performed to examine the effects of DHEA on atherogenesis in rabbits on high cholesterol-fed diets and in cultured vascular smooth muscle cells (VSMCs). Ox-LDL and DHEA were given to the VSMCs which were separately transiently transfected by the plasmid with or without the gene of cytochrome P450 aromatase (CYP19), which is the key enzyme in DHEA converting into testosterone and estradiol, and the expression level of MCP-1 and VCAM-1 were detected to judge whether the anti-atherogenic effects of DHEA is through the conversion of DHEA into testosterone and estradiol.
Materials and methods
Materials
Fetal bovine serum (FBS), culture medium M199, Dulbecco's modified Eagle's medium (DMEM)/F12 and Opti-MEM was obtained from Gibco, USA. DHEA was purchased from Fluka. All-trans retinoic acid was bought from Shangdong Liangfu Group Pharmaceutical Co, Ltd, China. Lipofectamine2000, primer and Trizol were supplied by Invitrogen. M-MLV reverse transcriptase, olig(dT), and real time polymerase chain reaction (PCR) kit were supplied by Toyobo. Enzyme-linked immunosorbent assay (ELISA) kit was obtained from Biosource. An EZNA Fastfilter Endo-free plasmid Maxi Kit was supplied by Omega. Ox-LDL and pEGFP-C3 were provided by the Department of Biochemistry and Molecular Biology, Tongji Medical College, Huazhong Science and Technology University. The plasmids of PCMV and PCMV-CYP19 were generously donated by Prof CONLEY (University of California-Davis California, USA).
Animals and diets
New Zealand White (NZW) rabbits were purchased from the Animal Experiment Center of Tongji Medical College, Huazhong Science and Technology University. Forty male New Zealand White rabbits (3 months old) and weighing 2.0–2.5 kg were individually caged and maintained in a controlled facility at 20±3°C with a 12-h light/dark cycle and with free access to water. The rabbits were divided into 5 groups (n=8 per group) and fed the following diets for 10 weeks. Group 1 received a high-cholesterol diet (regular rabbit diet with 1% cholesterol [HCD]). Group 2 received the HCD supplemented with 0.25% DHEA. Group 3 received the HCD supplemented with 0.25% DHEA and atRA (0.6 mg·kg−1·d−1). Group 4 received a regular diet supplemented with 0.25% DHEA. Group 5 received a regular diet.
Serum lipids determination
All rabbits were anesthetized with thiopental sodium and the blood samples (5 mL) were directly obtained from the heart. Collected serum was analyzed in an automatic blood chemical analyzer and the serum concentrations of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were obtained.
Histological evaluation of atherosclerosis
The descending thoracic aorta was then dissected longitudinally and separated into three portions. The first portion, a one-centimeter segment proximal to the outlet of the first intercostal artery was frozen in liquid nitrogen until further processing. The second portion, a segment between the first and the second intercostals artery, was paraffin-embedded and routinely processed, for histological examination. The third portion, a segment between the second and the seventh intercostals arteries, was fixed in 10% neutral buffered formalin for 1 day. The aorta was then placed in absolute propylene glycol for 2 min and stained with Sudan IV for 4 h. After washing, the extent of the Sudan IV-positive area was measured and expressed as a percentage of the internal surface using a computer-assisted morphometry system (HMIAS-2000 high definition Image analysis system, Qianping, Wuhan, China). The second portion of the aorta was cut into 4 mm sections. Parts of the sections were stained with basic fuchsin elastic stain and the most obvious intima hyperplasia was chosen to detect the parameters of the morphological change by light microscopy. The parameters were: intima and media thickness of the aorta and the ratio of intima and media.
MCP-1 and VCAM-1 mRNA expressions in rabbit artery
Total RNA was isolated from the first portion using TRIZOL reagent. MCP-1 and VCAM-1 mRNA expressions were determined by semiquantitative PCR, using GAPDH as an internal standard. The concentrations and purity of total RNA were calculated after spectrophotometric measurements at 260-nm wavelength. Each RNA sample (4 μg) was reverse transcribed into cDNA. 1 μL of cDNA was dissolved in 20 μL of a reaction mixture containing 2 μL of 10×PCR buffer, 0.4 μL of dNTP, 0.4 μL of up and downstream primers, 1.2 μL of MgCl2, 0.2 μL of Taq polymerase and distilled water. The PCR conditions of MCP-1, VCAM-1 and GAPDH genes are shown in Table 1. Amplification products obtained in PCR were electrophoretically separated on 2% agarose gel.
VCAM-1 protein expression in rabbit artery
Immunohistochemical analysis was performed on the second portion of the aorta for detection of VCAM-1, using rabbit polyclonal antibodies against rabbit VCAM-1 (1:200). Endogenous peroxidase was blocked with 0.3% H2O2 for 20 min. The secondary antibodies for immunostaining were biotin-conjugated goat anti-rabbit immunoglobulins.
VSMCs culture
The thoracic cavity was open under the aseptic condition and the thoracic aorta was obtained from male Sprague–Dawley rats of 4–6 weeks old had been decapitated. The aorta was put in phosphate-buffered saline (PBS) and the intima was scraped. VSMCs were cultured by the explant attached method18 and the complete medium of the cells contained M199 supplemented with 10% FBS and 0.03% glutamine. The VSMCs were fusiform and had peak and valley characteristics observed under the phase contrast microscope when growing to the confluence condition. Meanwhile, the monoclonal antibody of α-actin was used to identify the cells by immunohistochemistry. The 3−4 passages of VSMCs were used for the experiment. The purity of the cells was above 95%. Confluent VSMCs were digested by trypsin solution and adjusted to the density of 1.0×106 cells/mL for subculture. Parts of the cells were attached to cover glasses in incubation bottles for immunocytochemistry analysis. Twenty hours later, the medium were changed into DMEM/F12 serum-free medium and the cells were continued culturing for 24 h until the metabolic stage. The cells were then divided into 6 groups. Group 1 (normal control group): cells cultured in DMEM/F12 serum-free medium; group 2 (ox-LDL induced group): cells cultured in DMEM/F12 serum-free medium plus 50 mg/L ox-LDL; group 3 (ox-LDL and 50 μmol/L DHEA group): cells cultured in DMEM/F12 serum-free medium plus 50 mg/L ox-LDL, 50 μmol/L DHEA; group 4 (ox-LDL and 5 μmol/L DHEA group): cells cultured in DMEM/F12 serum-free medium plus 50 mg/L ox-LDL, 5 μmol/L DHEA; group 5 (ox-LDL and 0.5 μmol/L DHEA group): cells cultured in DMEM/F12 serum-free medium plus 50 mg/L ox-LDL, 0.5 μmol/L DHEA; group 6 (DHEA group): cells cultured in DMEM/F12 serum-free medium plus 5 μmol/L DHEA. The cells were collected 24 h later.
Transient transfection
Tranfection was carried out after determining the best transfection efficiency, which was caught when the density of the cells was 2.0×105 cells/mL and the ratio of DNA (μg) to Lipofectamine 2000 (μL) was 1:2 (Figure 1). And in preliminary transfection, other two enzymes besides CYP19, 3beta-hydroxysteroid dehydrogenase (3betaHSD) and a 17beta-hydroxysteroid dehydrogenase in synthesis of estradiol from DHEA were detected by semiquantitative PCR to ensure that DHEA could be transformed into estradiol after transfection. VSMCs were plated into 12-well format at the planting density of 2.0×105 cells/well the day before transfection, cultured in complete medium. Twenty hours later, the cells were washed several times and transfected separately with the plasmids of PCMV-CYP19 or PCMV. Another 24 h later, the medium was changed into Opti-MEM reduced serum medium and cultured for 2 h to make the cells synchronized. Then the cells transfected with PCMV-CYP19 or PCMV were divided into 3 groups: group 1 (control group): cells cultured in Opti-MEM reduced serum medium; group 2 (ox-LDL induced group): cells cultured in Opti-MEM reduced serum medium plus 50 mg/L ox-LDL; group 3 (ox-LDL and DHEA group): cells cultured in Opti-MEM reduced serum medium plus 50 mg/L ox-LDL, 5 μmol/L DHEA. Each group could be divided by the wells transfected with the plasmid of PCMV-CYP19 and the wells with PCMV. The cells and supernatant were collected 24 h later.
MCP-1 and VCAM-1 mRNA expressions in VSMCs
Total RNA was extracted from each group of VSMCs using TRIZOL. The cDNA reversed from total RNA of VSMCs was amplified by PCR and electrophoretically displayed on 2% agarose gel. The PCR conditions of MCP-1, VCAM-1 and β-actin genes were shown in Table 1 and the protocol was the same as before. Images of Goldwell-stained bands for MCP-1, VCAM-1 and β-actin cDNAs were scanned with the SQ9636 scanning system. The intensity of the bands (A) was densitometrically measured with the HMIAS-2000 high definition color medical analytic system. All MCP-1 and VCAM-1 signals were normalized to mRNA levels of the housekeeping gene β-actin and expressed as a ratio.
VCAM-1 and MCP-1 protein in VSMCs
The protein was detected by immunocytochemistry, Western blot and ELISA. Immunocytochemistry analysis: VSMCs, which were attached to cover glasses in incubation bottles were fixed with a mixture of acetone and ethanol (1:1) for 10 min. Immunocytochemical analysis was carried out by anti-VCAM-1. Results were determined using a multicolor image analysis system (HMIAS-2000).
Total cell extracts were subjected to Western blot analyses. Protein extracts were separated by SDS-PAGE using 7.5% polyacrylamide gels and electroblotted onto hydrophobic polyvinylidene difluoride membranes. After electroblotting, the membranes were blocked with 5% nonfat dry milk in 10 mmol/L Tris-HCl (pH 7.4) with 150 mmol/L NaCl and 0.1% Tween 20 (TTBS) for 1 h at 23°C. The membranes were then incubated with goat polyclonal antibodies against rat VCAM-1 overnight. After several washes, the membranes were incubated for 1 h with peroxidase-conjugated secondary antibody. The immunoreactive proteins were identified using an enhanced chemiluminescence reagent system, and analyzed with a gel documentation system (GDS8000, UVP, UK and HMIAS-2000).
ELISA analysis: The supernatant of transfected VSMCs was plated on 96-well plates that had been incubated with the first antibody. The detection was done according to the protocol of the ELISA kit. Plates were read on an ELISA reader at optical density (OD) 492 nm after blanking on rows stained only with second step antibody. Data represents the intensity of the samples (A).
Statistical analysis
The data were analyzed by the statistics software of SPSS12.0. Normal distributed results that were tested for normality are expressed as mean±SD obtained from at least three separate experiments in each group. Differences between groups were assessed by one-way ANOVA and Newman-Keuls multiple comparison test where appropriate. Significance was defined at P<0.05.
Results
Serum lipids
Changes in the serum lipid levels are shown in Table 2. After 10 weeks, there were no significant differences in triglyceride and HDL levels among the 4 experimental groups and the control group (group 5) over the course of the study. Group 1 (HCD group) had the highest TC, TG and LDL levels. Compared with this group, the addition of DHEA to the diet decreased LDL level and the addition of DHEA and atRA to the diet decreased TC and LDL levels. Group 3 had lower TC and LDL levels compared with group 2. There was no obvious difference between group 4 and 5.
Aortic fatty streak lesions
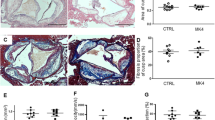
The fatty streak lesions of the thoracic aorta in each group were easily identified by staining with Sudan IV. Broad and fused fatty streak lesions were found in group 1, whereas small plaques were sparsely observed in group 2 and 3 (Figure 2) and the area of atherosclerotic lesions (fatty streaks and fibrofatty plaques) were measured (Table 3). The second portion of the thoracic aorta was stained with basic fuchsin elastic stain. The intima and media thickness of the aorta and the ratio of intima and media were calculated (Table 4). Histological examination of the aorta revealed intima thickening was mainly due to an accumulation of foam cells, and proliferation of smooth muscle cells in the intima and a deposition of extracellular matrix substances. The intima thickness of the aorta and the ratio of intima and media in group 1 were the highest. Group 2 had obvious decreases on the two indexes compared with group 1, though the thickness and the ration were higher than the baseline level (group 5). There was no significant difference between group 2 and 3, and group 4 and 5.
Aortic fatty streak lesions of each group in animal experiment. The descending thoracic aorta after be fixed in 10% neutral buffered formalin for 1 day was placed in absolute propylene glycol for 2 min and then stained with Sudan IV for 4 h. After washing, the fatty streak lesions were stained red. Arrow: the fatty streak lesion of each group. Group 1 was stained most seriously, almost the whole aorta was red. Group 2 and 3 were partly stained red, group 4 and 5 have no change after staining. Group 1: a regular rabbit diet plus 1% cholesterol and 3 % lard (HCD); group 2: 0.25% DHEA was incorporated into the diet of group 1; group 3: the diet of group 1 plus 0.25% DHEA and all-trans retinoic acid (0.6 mg·kg−1·d−1); group 4: 0.25% DHEA was incorporated into the regular diet; group 5: a regular diet. Fatty streaks were obvious in group 1, and less in group 2 and 3. No fatty steal could be seen in group 4 and 5.
MCP-1 and VCAM-1 mRNA levels in rabbit artery
Expression levels of MCP-1 and VCAM-1 mRNA in rabbit artery were displayed in Figure 3. Group 1 expressed the highest levels of both genes. Addition of DHEA to HCD decreased MCP-1 and VCAM-1 PCR products but there was no obvious difference after adding atRA. No difference was observed between group 4 and 5.
The electrophoretically results of MCP-1 and VCAM-1 mRNA in rabbit artery. GAPDH was used as an internal standard. Left, Representative electrophorogram. Right, Intensity of MCP-1 and VCAM-1 mRNA bands were counted with a bioimage analyzer and were normalized with intensity of GAPDH mRNA. (1) group 1, a regular rabbit diet plus 1% cholesterol and 3 % lard (HCD); (2) group 2, 0.25% DHEA was incorporated into the diet of group 1; (3) group 3, the diet of group 1 plus 0.25% DHEA and all-trans retinoic acid (0.6 mg·kg−1·d−1); (4) group 4, 0.25% DHEA was incorporated into the regular diet; (5) group 5, a regular diet. The mRNA expression of MCP-1 and VCAM-1 in group 1 was the highest among groups. Group 4 and 5 were the lowest. Group 2 and 3 had no significant difference. bP<0.05 vs group 1. eP<0.05 vs group 2.
VCAM-1 protein expression in rabbit artery
To establish that DHEA resists atherosclerosis in rabbit artery through inhibiting the secretion of VCAM-1, immunohistochemical analysis was performed (Figure 4). The HCD group had massive brown-yellow particles under the rabbits' aortic endothelium, prompting huge VCAM-1 protein secretion. Compared with this group, there were just basic secretion in group 4 and 5, and attenuation could be observed in group 2 and 3. However, there was no significant difference between group 2 and group 3.
Immunohistochemical results of VCAM-1 in rabbit artery. (A) group 1, a regular rabbit diet plus 1% cholesterol and 3 % lard (HCD); (B) group 2, 0.25% DHEA was incorporated into the diet of group 1; (C) group 3, the diet of group 1 plus 0.25% DHEA and all-trans retinoic acid (0.6 mg·kg−1·d−1); (D) group 4, 0.25% DHEA was incorporated into the regular diet; (E) group 5, a regular diet. Arrow: the brown-yellow particles under the endothelium which indicate VCAM-1 expression. Group 4 and 5 had only basic non-special expression.
MCP-1 and VCAM-1 mRNA levels in VSMCs
Three clear bands of each group of VSMCs stained by Goldwell were discovered under the uviol lamp: one was the MCP-1 gene of 440 bp, one was the VCAM-1 gene of 570 bp, and the third was the housekeeping gene β-actin of 173 bp based on the DNA marker. In untransfected VSMCs (Figure 5), compared with other groups, the expressions of MCP-1 and VCAM-1 mRNA of the ox-LDL induced group were conspicuously higher. When DHEA was added, the expression decreased evidently. There were no obvious difference between the DHEA group and the normal control group. In transfected VSMCs (Figure 6), the cells tranfected with PCMV-CYP19 or PCMV after being given 50 mg/L, ox-LDL had obviously considerable MCP-1 and VCAM-1 mRNA contents than any other groups. After being given 5 μmol/L DHEA, the expressions of MCP-1 and VCAM-1 mRNA decreased, the alteration coincided with the change in untransfected cells. However, there were no significant difference between the cells tranfected with PCMV-CYP19 and the cells tranfected with PCMV.
The electrophoretically results of MCP-1 and VCAM-1 mRNA in untransfected VSMCs. -actin was used as an internal standard. Left, Representative electrophorogram. Right, Intensity of MCP-1 and VCAM-1 mRNA bands were counted with a bioimage analyzer and were normalized with intensity of GAPDH mRNA. (1) the normal control group, with DMEM/F12 serum-free medium; (2) the ox-LDL induced group, 50 mg/L ox-LDL with DMEM/F12 serum-free medium; (3) the ox-LDL and 50 μmol/L DHEA group, 50 mg/L ox-LDL, 50 μmol/L DHEA with DMEM/F12 serum-free medium; (4) the ox-LDL and 5 μmol/L DHEA group, 50 mg/L ox-LDL, 5 μmol/L DHEA with DMEM/F12 serum-free medium; (5) the ox-LDL and 0.5 μmol/L DHEA group, 50 mg/L ox-LDL, 0.5 μmol/L DHEA with DMEM/F12 serum-free medium; (6) the DHEA group, 5 μmol/L with DMEM/F12 serum-free medium. The mRNA expression of MCP-1 and VCAM-1 in group 1 was the highest among groups. Group 2 and 4 were the lowest and had no significant difference. bP<0.05 vs group 2. eP<0.05 vs group 4.
The electrophoretically results of MCP-1 and VCAM-1 mRNA in transfected VSMCs. β-actin was used as an internal standard. Left, Representative electrophorogram. Right, Intensity of MCP-1 and VCAM-1 mRNA bands were counted with a bioimage analyzer and were normalized with intensity of GAPDH mRNA. (1) normal control group transfected with PCMV-CYP19; (2) ox-LDL induced group transfected with PCMV-CYP19; (3) ox-LDL and DHEA group transfected with PCMV-CYP19; (4) normal control group transfected with PCMV; (5) ox-LDL induced group transfected with PCMV; (6) ox-LDL and DHEA group transfected with PCMV. The mRNA expression of MCP-1 and VCAM-1 in group 1 was the highest among groups. Group 4 and 5 were the lowest. Group 2 and 3 had no significant difference. bP<0.05 vs group 2. eP<0.05 vs group 3.
VCAM-1 and MCP-1 protein expression in VSMCs
As displayed in Figure 7, all groups of cultured VSMCs express VCAM-1 protein. Compared with the normal control group, ox-LDL obviously enhanced VCAM-1 protein expression. Compared with the ox-LDL induced group, VCAM-1 expression decreased after DHEA was added in. There were no visible difference between the DHEA group and the normal control group. The results of Western blot analysis were same (Figure 8).
Immunocytochemical results of VCAM-1 in untransfected VSMCs (×200). (A) the normal control group, with DMEM/F12 serum-free medium; (B) the ox-LDL induced group, 50 mg/L ox-LDL with DMEM/F12 serum-free medium; (C) the ox-LDL and 50 μmol/L DHEA group, 50 mg/L ox-LDL, 50 μmol/L DHEA with DMEM/F12 serum-free medium; (D) the ox-LDL and 5 μmol/L DHEA group, 50 mg/L ox-LDL, 5 μmol/L DHEA with DMEM/F12 serum-free medium; (E) the ox-LDL and 0.5 μmol/L DHEA group, 50 mg/L ox-LDL, 0.5 mol/L DHEA with DMEM/F12 serum-free medium; (F) the DHEA group, 5 μmol/L with DMEM/F12 serum-free medium. Arrow: the brown-yellow particles in VSMCs which indicate VCAM-1 expression. Picture A and D had no VCAM-1 expression or just little expression.
Western blot analyses of VCAM-1 in untransfected VSMCs. (1) the normal control group, with DMEM/F12 serum-free medium; (2) the ox-LDL induced group, 50 mg/L ox-LDL with DMEM/F12 serum-free medium; (3) the ox-LDL and 50 μmol/L DHEA group, 50 mg/L ox-LDL, 50 μmol/L DHEA with DMEM/F12 serum-free medium; (4) the ox-LDL and 5 μmol/L DHEA group, 50 mg/L ox-LDL, 5 μmol/L DHEA with DMEM/F12 serum-free medium; (5) the ox-LDL and 0.5 μmol/L DHEA group, 50 mg/L ox-LDL, 0.5 μmol/L DHEA with DMEM/F12 serum-free medium; (6) the DHEA group, 5 μmol/L with DMEM/F12 serum-free medium.
The supernatant of transfected VSMCs was extracted from the wells 24 h after being given the drugs. The MCP-1 protein was detected following the protocol of the ELISA kit. Results are displayed by the intensity (A) at 492 nm detected by the ELISA reader (Figure 9). No matter what plasmid the cells were transfected with, the ox-LDL induced group had the largest MCP-1 quantity, and DHEA apparently inhibited MCP-1 protein expression. The difference in the expression of MCP-1 protein between the ox-LDL and DHEA group that was transfected with the plasmid of PCMV-CYP19 or with the plasmid of PCMV was not clearly observed.
The expression of MCP-1 protein detected by ELISA in transfected VSMCs. (1) normal control group transfected with PCMV-CYP19; (2) ox-LDL induced group transfected with PCMV-CYP19; (3) ox-LDL and DHEA group transfected with PCMV-CYP19; (4) normal control group transfected with PCMV; (5) ox-LDL induced group transfected with PCMV; (6) ox-LDL and DHEA group transfected with PCMV. bP<0.05 vs group 2. eP<0.05 vs group 3.
Discussion
This study showed that dietary treatment of rabbits with high-cholesterol diets made a successful atherosclerotic model, and 0.25% DHEA could inhibit the development of atherosclerosis. However, the serum lipid levels had little difference among these groups with or without DHEA. DHEA may have some effects on the plasma lipid profile through the peroxisomes or related enzymes19. However, the likelihood of such an effect is slight, because DHEA treatment did not change the serum lipid profile in this study; it seemed there might be other way to anti-atherosclerosis. The attenuation of atherogenesis in rabbits given HCD and 0.25% DHEA compared with rabbits given just HCD is paralleled with the expression of MCP-1 and VCAM-1 in those two groups, no whether the gene detections or the immunohistochemical analysis results. Furthermore, the variation of MCP-1 and VCAM-1 was in coincidence. These results all suggest that DHEA has anti-atherogenic function, which is closely related to the expression of MCP-1 and VCAM-1.
Ox-LDL has been implicated as an important trigger and sustaining element of atherosclerotic lesions. LDL oxidized by free radicals derived from activated macrophages, endothelial and smooth muscle cells are considered as the major atherogenic lipoproteins20. In VSMCs experiment of this study, the function of DHEA to VSMCs, which were induced by ox-LDL was observed by detecting the expressions of MCP-1 and VCAM-1. The dose-effect relationship of DHEA was also observed. The mRNA levels of MCP-1 and VCAM-1 in untranfected VSMCs was significantly up-regulated after 24 h induced by 50 mg/L ox-LDL. However, the expressions were obviously downregulated after the addition of DHEA and ox-LDL. This indicates that DHEA has the ability to inhibit ox-LDL induced expression of MCP-1 and VCAM-1 in VSMCs. And the different dose of DHEA had different anti-atherogenic effect. 50 μmol/L DHEA had little effect, and 0.5 μmol/L DHEA had the effect just little than 5 μmol/L DHEA. These results reflect that high concentration of DHEA did not have high anti-atherogenic effect but also would reduce this effect. And low concentrations of DHEA could not exert enough anti-atherogenic effects. DHEA alone did not change the expressions of MCP-1 and VCAM-1 mRNA in VSMCs compared with the normal control group. This demonstrated that DHEA has no effect on normal VSMCs. The results of MCP-1 and VCAM-1 protein reflected the difference of 6 groups as same as in gene level. All of these suggested that DHEA executes its anti-atherosclerotic effect by influencing the expression of MCP-1 and VCAM-1.
Atherosclerosis is characterized by intimal lesions called atheromas or fibrofatty plaques that protrude into the vascular lumen, weaken the underlying media, leading to a number of complications21. The initiating event of atherosclerosis is an endothelial dysfunction followed by smooth muscle proliferation and architectural disruption of the vessel22. Monocytes are then recruited by endothelial cells and invade the sub-intimal space by a complex mechanism characterized by the enhanced expression of chemoattractant interleukins, adhesion molecules.
DHEA and its sulfate DHEAS are weak but highly abundant adrenal androgens that in both men and women. Although DHEA and DHEAS have few intrinsic androgenic actions, recent studies have found that they have widespread effects. DHEA biosynthesis decreases with age and the resulting low plasma levels might be associated with the development of diseases linked to aging such as atherosclerosis23. Many researches investigated that DHEA may be an endogenous anti-atherogenic factor. It is speculated to have a wide application perspective on treatment and prevention of athrosclerosis, although the mechanism of action remains unclear. As we all know, atherosclerosis is recognized as an inflammatory process. So there are some researchers thought that DHEA might have a protective role against atherosclerosis through mediating inflammatory action. López-Marure evaluated the effects of DHEA on several molecules involved in the inflammatory response. The results suggest that DHEA inhibits the expression of molecules involved in the inflammatory process in endothelial cells activated with ox-LDL, such as reactive oxygen species (ROS) production, intracellular adhesion molecule-1 (ICAM-1), platelet endothelial cell adhesion molecule-1 (PECAM-1)24. Our study proposed that a putative mechanism of DHEA anti-atherosclerotic effect may be the interference with the adhesion of inflammatory cells as a result of a lower expression of cell adhesion molecules and chemoattractant interleukins induced by DHEA. Epidemiological studies showed an inverse relationship between cardiovascular mortality and plasma DHEA(S) levels in men25, 26. There have been some reports about the clinical application of DHEA27, 28.
As DHEA has so many functions, a lot of studies were developed to determine its precise mechanism. However, the possibility of it having biological actions in its own right has been largely overlooked, in that it has generally been assumed that its actions reflect conversion to androgenic and estrogenic metabolites. Evidence in favor of this assumption includes the presence of many enzymes responsible for the bioconversion of DHEA including aromatase cytochrome P450 (P450 arom), which can aromatize androgens into estrogens in many peripheral tissues and perhaps also in VSMCs. This is supported by the findings of Hayashi et al who demonstrated that the anti-atherogenic effects of DHEA in ovariectomized female rabbits can be partially (50%) blocked by the aromatase inhibitor fadrozole29. Furthermore, numerous studies have shown that DHEA administration results in increased levels of androgens and estrogens and that prolonged pretreatment periods with DHEA are required before an effect is observed, suggesting that the actions of DHEA may result from the production and subsequent action of estrogenic or androgenic metabolites arising from DHEA conversion.
To resolve the ambiguity of whether DHEA plays an anti-atherogenic action by conversion to androgenic and estrogenic metabolites, CYP19 was transfected into VSMCs and the expression of MCP-1 and VCAM-1 gene and protein were detected in the cells that were given DHEA and ox-LDL. P450 arom is the key enzyme that catalyzes the conversion of C19 steroids to estrogens and is encoded by a single gene (CYP19) in the human genome. It has been reported that DHEA converts into androgen and estrogen through the CYP19 coded enzyme P450 arom. So the activity of P450 arom is important to the function of DHEA to certain tissues. Consequently, the groups induced by ox-LDL had the most quantities of MCP-1 and VCAM-1, the normal control groups had the lowest MCP-1 and VCAM-1 levels, but the corresponding groups had no conspicuous difference with each other. These results suggested that the cells transfected with PCMV-CYP19 and PCMV had the same conditions and ox-LDL successfully induced the cells into atherosclerotic foam cells. The addition of 5 μmol/L DHEA to the medium reduced the expression level of MCP-1 and VCAM-1, which indicated that DHEA has an anti-atherosclerotic effect. The comparison between the ox-LDL and DHEA group that was transfected with the plasmid of PCMV-CYP19 or PCMV had no observed difference regardless of the gene or protein level. This was not consistent with the hypothesis that if DHEA plays an atheroprotective effect through conversion, the cells tranfected with PCMV-CYP19 would enhance its conversion and the expressions of MCP-1 and VCAM-1 would be apparently attenuated.
It has been indicated that P450 arom has many promoters and in vascular smooth muscle cells and THP-1 cells, the main promoter is exon I.6. Retinoic acid receptor can increase the expression of P450 arom through promoter after it has been activated30, 31. All trans-retinoic acids (atRA) are activators of retinoic acid receptors. Maiti et al indicated that atRA can induce the expression of P450 arom32. Some researchers have indicated that it can reduce intimal thickening after balloon angioplasty in atherosclerotic rabbits33. Therefore, to further research the atheroprotective properties of DHEA with conversion, atRA was designed in an animal experiment. As stated previously, atRA would promote the effect of DHEA if it converted into androgen and estrogen. However, the results of this study did not find any obvious advanced function of atRA to the anti-atherogenesis of DHEA in NZW rabbits without intimal mechanical injury after being fed with HCD and DHEA. There were no significant differences in serum lipid levels, aortic fatty streak lesions and the expressions of MCP-1 and VCAM-1 in groups 2 and 3. This suggested that DHEA might not play the anti-atherogenic function by converting into androgen or estrogen.
Recently, the possibility of direct actions of DHEA has been increasingly canvassed. High-affinity binding sites for DHEA were identified in bovine endothelial cells suggesting that DHEA may exhibit direct vascular effects34. Simoncin et al are of the same opinion in that there might be receptor-mediated anti-atherogenic action of DHEA independent of the metabolism and conversion of it in peripheral tissue35. DHEA has also been shown to activate endothelial nitric oxide synthase (eNOS) in endothelial cells34, 36 potentially via a G-protein coupled plasma membrane receptor33. Its genomic and nongenomic effects are not blocked by antagonists of the estrogen, progesterone, glucocorticoid, or androgen receptors36. Similarly, DHEA affects extracellular signal-regulated kinase 1 (ERK-1) phosphorylation in human vascular smooth muscle cells independently of androgen and estrogen receptors37. Results from Zapata et al show that DHEA inhibits the proliferation of HUVEC in a dose-dependent manner. This anti-proliferative effect is associated with cell cycle arrest in the G1 phase, an increase in the active form of RB protein, an increase in the expression of p21 and p53, and the absence of apoptotic cell death38. As atherosclerosis is an inflammation, some researchers found that it could reduce interleuking-6 (IL-6), IL-10, serum amyloid P substance, and serum immunoglobulin (Ig) in aged mice treated with DHEAS39, 40. Dehydroepiandrosterone delays LDL oxidation in vitro and attenuates several oxLDL-induced inflammatory responses in endothelial cells, such as ICAM-1, PECAM-1 and VCAM-1; DHEA inhibited ox-LDL-induced ROS production; and DHEA increased AP-1 translocation24. Dehydroepiandrosterone inhibits the tumor necrosis factor-α-induced inflammatory response in human umbilical vein endothelial cells41. Therefore, its potential anti-inflammatory properties should be evaluated for the treatment of chronic inflammatory diseases such as atherosclerosis.
Many possible mechanisms might be involved in the atheroprotective effect of DHEA. The results of our study indicated that the anti-atherosclerotic effect of DHEA has no obvious relationship with the bioconversion to androgenic and estrogenic metabolites, but it does have a strong possibility of having its own bioactivity in VSMCs. Further investigations are required to ascertain the mechanism of DHEA in anti- atherogenesis.
Author contribution
Prof Qiu-rong RUAN designed research; Heng-hui CHENG and Xiao-jing HU performed research; Prof Qiu-rong RUAN contributed new analytical tools and reagents; Heng-hui CHENG and Xiao-jing HU analyzed data; Heng-hui CHENG wrote the paper.
References
Parker CR . Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 1999; 64: 640–7.
Zdrojewicz Z, Ciszko B . Dehydroepiandrosterone (DHEA)-structure, clinical importance and the role in human body. Postepy Hig Med Dosw 2001; 55: 835–54.
Williams JR . The effects of dehydroepiandrosterone on carcinogenesis, obesity, the immune system, and aging. Lipids 2000; 35: 325–31.
PorŠovÁ-dutoit I, ŠulcovÁ J, StÁrka L . Do DHEA/DHEAS play a protective role in coronary heart disease? Physiol Res 2000; 49 (Suppl 1): S43–S56.
Fredrick CWW, Arnold VE . Androgens and coronary artery disease. Endocrine Rev 2003; 24: 183–217.
Dimitrakakis C, Zhou J, Bondy CA . Androgens and mammary growth and neoplasia. Fertil Steril 2002; 77: S26–S33.
Notelovitz M . Androgen effects on bone and muscle. Fertil Steril 2002; 77: S34–S41.
Hayashi T, Muto E, Kano H, Asai Y, Thakur NK, et al. Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: the possible role of nitric oxide. Arterioscler Thromb Vasc Biol 2000; 20: 782–92.
Yoneyama A, Kamiya Y, Kawaguchi M, Fujinami T . Effects of dehydroepiandrosterone on proliferation of human aortic muscle cells. Life Sci 1997; 60: 833–8.
Ross R . Cell biology of atherosclerosis. Annu Rev Physiol 1995; 57: 791–804.
Takahashi M, Ikeda U, Masuyama J, Kitagawa S, Kasahara T, Saito M, et al. Involvement of adhesion molecules in human monocyte adhesion to and transmigration through endothelial cells in vitro. Atherosclerosis 1994; 108: 73–81.
Valente AJ, Rozek MM, Sprague EA, Schwartz CJ . Mechanisms in intimal monocyte-macrophage recruitment: a special role for monocyte chemotactic protein-1. Circulation 1992; 86 (Suppl III): III-20–III-25.
Kuijpers TW, Harlan JM . Monocyte-endothelial interactions: insights and questions. J Lab Clin Med 1993; 122: 641–51.
Iivama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 1999; 85: 199–207.
Walker LN, Reidy MA, Bowyer DE . Morphology and cell kinetics of fatty streak lesion formation in the hypercholesterolemic rabbit. Am J Pathol 1986; 125: 450–9.
Parhami F, Fang ZT, Fogelman AM, Andalibi A, Territo MC, Berliner JA . Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest 1993; 92: 471–8.
Nelken NA, Coughlin SR, Gordon D, Wilcox JN . Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest 1991; 88: 1121–7.
Freshney RI . Culture of Animal Cells: A Manual of Basic Technique. New York: Alan R. Liss, Inc; 1987.
Rao MS, Ide RE, Subbarao V, Reddy AJ . Dehydroepiandrosterone induced peroxisome proliferation in the rat: evaluation of sex differences. Proc Soc Exp Biol Med 1994; 20: 186–90.
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL . Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989; 320: 915–24.
Brown AJ . Atherosclerosis: Cell biology and lipoproteins. Curr Opin Lipidol 2000; 11: 667–9.
Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM . The pathogenesis of atherosclerosis: an overview. Clin Cardiol 1991; 14(2 Suppl 1): 11–6.
Barret-Connor E, Goodman-Gruen D . The epidemiology of DHEAS and cardiovascular disease. Ann NY Acad Sci 1995; 774: 259–70.
López-Marure R, Huesca-Gómez C, Ibarra-Sánchez Mde J, Zentella A, Pérez-Méndez O . Dehydroepiandrosterone delays LDL oxidation in vitro and attenuates several oxLDL-induced inflammatory responses in endothelial cells. Inflamm Allergy Drug Targets 2007; 6: 174–82.
Vatalas IA, Diony-Asteriou A . Adrenal C19 steroids and lipoprotein levels in healthy men. Nutr Metab Cardiovasc Dis 2001; 1: 388–93.
Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, et al. The plasma levels of dehydroepiandrosterone sulfate are decreased in patients with chronic heart failure in proportion to the severity. J Clin Endocrinol Metab 2000; 85: 1834–40.
Silvestri A, Gambacciani M, Vitale C, Monteleone P, Ciaponi M, Fini M, et al. Different effect of hormone replacement therapy, DHEAS and tibolone on endothelial function in postmenopausal women with increased cardiovascular risk. Maturitas 2005; 50: 305–11.
Callies F, Fassnacht M, van Vlijmen JC, Koehler I, Huebler D, Seibel MJ, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab 2001; 86: 1968–72.
Hayashi T, Esaki T, Muto E, Kano H, Asai Y, Thakur NK, et al. Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: The possible role of nitric oxide. Arterioscler Thromb Vasc Biol 2000; 20: 782–92.
Bouraima H, Hanoux V, Mittre H . Expression of the rabbit cytochrome P450 aromatase encoding gene uses alternative tissue-specific promoters. Eur J Biochem 2001; 268: 4506–12.
Shozu M, Zhao Y, Bulun SE, Simpson ER . Multiple splicing events involved in regulation of human aromatase expression by a novel promoter, I.6. Endocrinology 1998; 139: 1610–7.
Chen J, He B, Zheng D, Zhang S, Liu J, Zhu S . All-trans retinoic acid reduces intimal thickening after balloon angioplasty in atherosclerotic rabbits. Chin Med J (Engl) 1999; 112: 121–3.
Maiti S, Chen X, Chen G . All-trans retinoic acid induction of sulfotransferases. Basic Clin Pharmacol Toxicol 2005; 96: 44–53.
Liu D, Dillon JS . Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma 386 seminars in reproductive medicine/volume 22, number 4 2004 membrane receptor coupled to Galpha(i2,3). J Biol Chem 2002; 277: 21 379–88.
Furutama D, Fukui R, Amakawa M, Ohsawa N . Inhibition of migration and proliferation of vascular smooth muscle cells by dehydroepiandrosterone sulfate. Biochim Biophys Acta 1998; 1406: 107–14.
Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR . Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology 2003; 144: 3449–55.
Williams MR, Ling S, Dawood T, Hashimura K, Dai A, Li H, et al. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J Clin Endocrinol Metab 2002; 87: 176–81.
Zapata E, Ventura JL, De la Cruz K, Rodriguez E, Damián P, Massó F, et al. Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial cells by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgen- and estrogenreceptor independent. FEBS J 2005; 272: 1343–53.
Daynes RA, Araneo BA, Ershler WB, Maloney C, Li GZ, Ryu SY . Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol 1993; 150: 5219–30.
Spencer NF, Norton SD, Harrison LL, Li GZ, Daynes RA . Dysregulation of IL-10 production with aging: possible linkage to the age-associated decline in DHEA and its sulfated derivative. Exp Gerontol 1996; 31: 393–408.
Gutiérrez G, Zapata E, Montiel A, Reyes E, Montaño LF, et al. Dehydroepiandrosterone inhibits the TNF-alpha-induced inflammatory response in human umbilical vein endothelial cells. Atherosclerosis 2007; 190: 90–9.
Acknowledgements
Project supported by grants from the National Natural Science Foundation of China (No 30300135 and 30570725), Natural Science Foundation of Hubei Province (No 2005ABA166).
We thank Alan J CONLEY for providing plasmid and Shou-hua YANG for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Hh., Hu, Xj. & Ruan, Qr. Dehydroepiandrosterone anti-atherogenesis effect is not via its conversion to estrogen. Acta Pharmacol Sin 30, 42–53 (2009). https://doi.org/10.1038/aps.2008.2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2008.2
Keywords
This article is cited by
-
Discovery of novel aromatase inhibitors using a homogeneous time-resolved fluorescence assay
Acta Pharmacologica Sinica (2014)
-
DHEA supplementation in Menopause
Current Obstetrics and Gynecology Reports (2014)