Abstract
Here we report the use of unique organic−inorganic hybrid materials composed of octa-substituted polyhedral oligomeric silsesquioxane (POSS) cores as ionic liquid (IL) crystals. These materials have a wide temperature range in which they exist in liquid crystal (LC) phase because of the stabilizing effect of the POSS core. We synthesized ion pairs composed of alkyl chain-substituted imidazolium and carboxylates of various lengths that were or were not connected to the POSS core; then the thermal properties of these materials were investigated. It was found that both ion salts and the octadecyl-substituted imidazolium ion pairs with or without connection to POSS could form LCs. Interestingly, the LC phase of the POSS-tethered ion salts was maintained until decomposition. In contrast, the octadecyl-substituted imidazolium ion salts that were not tethered to the POSS core showed a clearance point during heating. The highly symmetric structure of POSS contributes not only to the suppression of the molecular motion of the ion salts, but also results in the formation of regular structures, leading to thermally stable, thermotropic IL crystals.
Similar content being viewed by others
Introduction
Ionic liquid (IL) crystals are highly relevant and are being heavily researched. The unique characteristics, especially their novel optical properties, of liquid crystals (LCs) have been reported previously.1, 2 In addition, by using electrostatic interactions originating among ionic moieties, robust chiral structures can be produced.3, 4 Even a small quantity of chiral ionic sources can efficiently induce enantiomeric structures in a bulk sample, and ionic moieties can be assembled to form regularly ordered structures.5, 6 These well-ordered ionic moieties are expected to work as efficient cation carriers and scaffolds for ordering cations. These materials, which comprise closely assembled ionic species, can exhibit interesting optical and magnetic properties. Thus, new molecular designs for preparing thermally stable LCs composed of ionic species are needed to produce advanced functional materials.
The concept of supported IL phases (SILPs) has been recently proposed. Several materials based on SILPs have exhibited important characteristics such as high ion conductivity and compatibility with organic reaction media; these materials have been used as templates for the formation of nanostructures.1 Silica supports improve the thermal stability of LCs.7, 8 Carmichael et al.9 have synthesized and explored silicon wafer-assisted SILP materials. They created layers with thicknesses between 10 and 21 nm of an ionic LC with [C18C1Im][PF6] on the planar wafer surface and found that the ionic LC formed a mesophase. Inspired by their work, Wasserscheid et al.7 fabricated SILP materials using silicon nanoparticles and used them as supports for preparing mesoporous structures that could act as effective gas-phase catalysts.8 To construct advanced materials based on the concept of SILP materials, their properties must be fine-tuned according to preprogramed designs at the molecular level. A wide variety of polyhedral oligomeric silsesquioxane (POSS)-based functional materials have been developed using ionic species.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 We recently reported the synthesis of POSS-based ILs, and we investigated their thermal properties.25, 26 By tethering multiple ion pairs composed of imidazolium cations and carboxylates to the vertices of POSS cores or by simply adding POSS molecules with acid groups, the melting temperatures of the ion pairs decreased and their thermal stabilities increased.25, 26, 27, 28 In the crystalline state, the POSS core can isolate each ion pair. As a result, the intermolecular interactions are reduced, and thus the melting temperatures are decreased. In the liquid state, the ion pairs are distributed in a spherical conformation as a result of their tethering to the POSS core. Therefore, their molecular symmetry can be improved and their thermal stability can be enhanced. Finally, we reported on the synthesis of room temperature ILs using POSS.25 Our interests have shifted, and we would like to exploit the thermal properties of POSS for realizing optical materials. In particular, we aim to construct unique optical materials based on SILP materials that utilize the structural features of POSS.
Herein, we report the extension of the temperature range of the LC phase by the POSS core. Ion pairs of carboxylate and imidazolium with various alkyl chain lengths were prepared, and the influence of the length of the alkyl chains and their tethering to the POSS core on the thermal properties of the ion pairs was investigated. Initially, the ion salts with the octadecyl alkyl chains at the imidazolium moiety exhibited LC phases. Significantly, the isotropic phase of the obtained POSS ion salts was not detected until decomposition occurred during the heating process. In other words, the temperature range of the LC phase was extended by the POSS core. In contrast, the ion salts that were not tethered to a POSS core showed a clearance point during heating. We propose that the highly symmetric structure of the POSS core should contribute not only to the suppression of the molecular motion of the ion salts but also to the formation of regular structures. To the best of our knowledge, this is the first time that a significant enhancement of LC formation has been detected using the structural characteristics of a molecular cube that imparts the IL crystals with unique thermal stabilities.
Experimental procedure
General procedure for the preparation of ILs
The desired equivalent moles of the bromide anion to the carboxyl groups were converted into the hydroxylic form using an anion exchange resin (Amberlite–IRA400, Sigma-Aldrich Inc., St Louis, MO, USA) in water and neutralized by suspension in methanol (2 l). The aqueous solution was concentrated using a rotary evaporator, and the residual liquid was freeze-dehydrated to give the white solid. The solid was dried in vacuo and stored in a glove box.
Differential scanning calorimetry (DSC)
DSC thermograms were produced on a SII DSC 6220 instrument (Seiko Instruments Inc., Chiba, Japan) using ∼10 mg of sample. The sample on the aluminum open pan was cooled to –130 °C at the rate of 10 °C min−1 under flowing nitrogen (30 ml min−1) and then heated from –130 to 80 °C at the same rate. The glass transition (Tg) and melting temperatures (Tm) were determined as the onset of the second curves to eliminate heat history. The fusion enthalpies (ΔHfus) were calculated from the areas of the endothermic peaks in the first cycle with the completely crystallized samples soaked in liquid nitrogen before measurement. To collect the transition temperatures of the mesophases, the measurements were monitored at a rate of 5 °C min−1. The transition temperatures (Tlc-iso) were evaluated from the second heating curves.
Thermogravimetric analysis (TGA)
TGA was performed on an EXSTAR TG/DTA6220 (Seiko Instrument, Inc.) at a heating rate of 10 °C min−1 up to a temperature 900 °C under flowing nitrogen (200 ml min−1). Residual water was removed by keeping the platinum pan at 110 °C for 1 h before the curve profiling was completed. The decomposition temperatures (Td) were determined from the onset of the weight loss.
Results and discussion
Smaller fusion entropies were obtained during the melting process for POSS-tethered ion salts than for ion salts without POSS.25, 26 Thus, it is likely that the molecular distribution of the ion pairs that are tethered to the POSS core in the crystals remain the same after melting, meaning that a star-shaped structure might be maintained before and after melting. Based on these presumptions, we aimed to align the POSS molecules to form regular structures in the liquid phase. By elongating the alkyl chains of the imidazolium cation, the hydrophobic interactions among the ion pair moieties should be enhanced, leading to the formation of well-aligned, high-dimensional structures in the POSS-based ILs. A series of POSS-tethered ion pairs with longer imidazolium cation alkyl chains were synthesized (Scheme 1; the detailed procedures and characterization data are shown in the Supporting Information). During anion exchange, the 1-alkyl-3-methylimidazolium bromides were transformed to the hydroxide form, and the desired products were prepared via neutralization with POSS-(COOH)8.25 After lyophilization, the products obtained were either colorless and transparent liquids or white solids. To prohibit coloration, all procedures were carried out without heating. We also prepared the ion pair Arm-CnIm and used it to evaluate the effect of the connection to the POSS core on the thermal properties of the materials.
The 29Si nuclear magnetic resonance spectra of all of the samples containing the POSS moiety exhibited a single peak at ∼66 p.p.m. that was assigned to the T8 POSS structure.25, 26 These data indicate that the POSS cage is not significantly degraded in the final products. The integration of the peaks in the 1H nuclear magnetic resonance spectra indicates the formation of 1:8 ion pairs of POSS-(COOH)8 and imidazolium cation. All samples were stored in a glove box under an argon atmosphere, and the water abundance was held below 1.5 wt. % as determined by the Karl Fischer method. The concentration of residual bromide ion was lower than the detectable level as determined by elemental analysis. Therefore, we concluded that all products were sufficiently pure.
Initially, the thermal stability of the synthetic compounds against pyrolysis was investigated using TGA. The Td, evaluated from the onsets of the TGA profiles, are summarized in Figure 1a (values are listed in Table S1 in the Supporting Information). It was found that the Td values for the POSS-CnIm samples were higher than those for the Arm-CnIm samples with the same alkyl chain length. When the length of the alkyl chain of the imidazolium cation was increased, the Td values of the POSS-CnIm samples gradually decreased. Conversely, those of the Arm-CnIm samples remained constant. The decomposition fragment of the molecule can be determined by evaluating the vertical bars in these profiles (from the weight loss to the molecular weight).25, 26 As a result, the decomposition process was explored. According to these decomposition mechanisms, the degradation should occur at the imidazolium ion pairs. These results indicate the stabilizing effect of the POSS core on the Td regardless of the alkyl chain length of the imidazolium moiety. In particular, the Td values were higher for the POSS salts with long alkyl chains, in which the POSS core did not greatly influence the molecular stabilization, than for the Arm salts. Regular structures, originating from the cubic core, might be formed; these structures may increase the stability of the system by limiting the thermal motion of the remote alkyl chains.
The Tm was determined from the peak top in the second cycle of DSC analysis (heating rate=10 °C min−1, Figure 1b (values are listed in Table S2 in the Supporting Information)). Most samples exhibited endothermic peaks below 100 °C. Therefore, they were classified as ILs.29 The POSS moiety can decrease the Tm values of the ion salts; this phenomenon can be seen by comparing the Tm values of ILs with the same alkyl chain lengths (POSS-CnIm and Arm-CnIm). These data can be explained by noting that the cubic core can inhibit molecular interactions between the ion salts.25, 26 In particular, for the POSS-CnIm samples, the alkyl chain length did not significantly influence the Tm values (for below n=14). POSS-C16Im can be categorized as a room temperature IL (an IL with a Tm below 25 °C). Conversely, Arm-CnIm (alkyl length, n=8) showed the lowest melting temperature, and the introduction of a longer alkyl chain (more than n=10) caused the Tm values to significantly increase. In general, such Tm dependencies have been observed in other types of ILs, and the lowest melting temperature was typically found for systems with alkyl chain lengths between n=2 and n=8.30 The Tm data, along with the TGA results, imply that the POSS core could significantly dominate the distributions of ion pairs. Thereby, the formation of the most thermally stable structure should be inhibited. Notably, the Tm value of the POSS salt with the C18 alkyl chains, where the POSS core does not likely influence the system to the same extent, was still lower than that of the Arm salt. This result implies that regular structures are formed. Tm values could not be reliably obtained for POSS-C8Im and POSS-C10Im because of their extremely poor crystallinities; unstable metastable states also exist in these structures.
The fusion enthalpies and entropies (ΔHfus and ΔSfus) for the single imidazolium molecule with POSS-C18Im and Arm-C18Im were evaluated from the areas of the endothermic peaks observed in the DSC profiles. Interestingly, smaller thermodynamic parameters (ΔHfus=30 kJ mol–1, ΔSfus=96 J mol–1 K–1) were obtained for the POSS-C18Im sample than for the Arm-C18Im sample (ΔHfus=65 kJ mol–1, ΔSfus=196 J mol–1 K–1). These thermal data are typical for POSS ILs.25, 26 The cubic silica core can isolate each ion pair. In addition, the star-shaped structure of the whole molecules comprising the POSS ILs should make interactions among ion pairs unfavorable. Smaller ΔHfus values were observed, leading to decreases in the Tm. Moreover, the star-shaped structure likely reduces the conformational variety of the ion pairs because of its high symmetry even in the liquid phase. Thus, thermal stability toward pyrolysis can be acquired. Such thermal characteristics, which originate from the POSS core, can be obtained for the C18Im salts. It is likely that the regular structure transmits the effect of the POSS core through the material.
Using DSC analysis, the phase transition behaviors of the salts containing C18Im were investigated (Figure 2). To collect a high resolution profile, the temperature was changed slowly (5 °C min−1). Although the endothermic peak associated with the melting of the Arm-C18Im samples was unclear, an endothermic transition was observed at 83 °C (Tlc-iso) after melting (Figure 2a). In contrast, the POSS-C18Im samples presented a clear endothermic transition at 45 °C that could be attributed to melting, and these samples did not show any other transitions until the decomposition temperature (Figure 2b). As summarized in Figure 2c, and in light of the Tms included in Figure 1, it is likely that the POSS-C18Im sample maintained its mesophase until the decomposition temperature and showed mesophase stability over a much wider temperature range (ΔT=163 °C) than the Arm-C18Im sample (ΔT=24 °C). Therefore, the connection of the ion salts to the POSS core greatly enhances the mesophase stability of ILs (higher mesophase stability, lower melting temperature). Polarized optical microscopy was performed on the samples containing C18Im (Figure 3). Arm-C18Im exhibited a focal-conic fan-like texture at 40 °C. Conversely, POSS-C18Im showed a mesophase of broken or small focal-conic fan-like texture. In particular, the mesophase of POSS-C18Im was maintained even at 100 °C. These results indicate that both Arm-C18Im and POSS-C18Im formed smectic mesophases. In particular, because POSS-C18Im shows higher thermal stability compared with Arm-C18Im, it is likely that the connection of the ion salts to POSS can extend the temperature region in which the samples act as thermotropic LCs.
Differential scanning calorimetry (DSC) profiles of (a) Arm-C18Im and (b) POSS-C18Im. (c) Summary of the phase transition temperatures of the ion salts, including the Tms, as shown in Figure 1. POSS, polyhedral oligomeric silsesquioxane; Td, decomposition temperature; Tm, melting temperature.
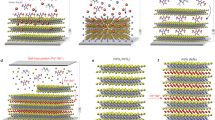
Variable temperature powder X-ray diffraction experiments were also executed to estimate the structures of the LCs (rate=1 °C min−1, Figure 4). In the Arm-C18Im mesophase, at 60 °C, two pairs of diffraction peaks were observed (at 5.11° and 7.70°); these peaks were attributed to the (02) and (03) peaks (d-spacing=34.4 Å) derived from the smectic layer distance (Figure 4a). Moreover, a wide-angle, broad halo peak was also observed at ∼20.0° (d-spacing=4.4 Å). This peak could be attributed to the molten alkyl chains. In contrast, in the POSS-C18Im mesophase, one diffraction peak was observed at 4.30°, and a broad halo was observed at ∼19.7° (d-spacing=4.5 Å) at 100 °C (Figure 4b). In small-angle X-ray scattering experiments (Figure 4c), two pairs of diffraction peaks (at q=1.528 and 3.06 nm−1 (d-spacing=41.1 Å)) were observed; these peaks were derived from the smectic layer distance. These values are consistent with the X-ray diffraction peaks attributed to the (02) diffraction. The extension of the d-spacing determined from the (02) diffraction can be explained by considering the size of the POSS core. By inserting the silica cube (∼5 Å in diameter), the layer distance was expanded (Figure 4d). Thus, a larger value was obtained.
Variable temperature powder X-ray diffraction (VT-PXRD) patterns of (a) Arm-C18Im at 60 °C (black line) and 30 °C (gray line) and (b) POSS-C18Im at 100 °C (black line) and 30 °C (gray line). (c) One-dimensional small-angle X-ray scattering (1D SAXS) patterns of POSS-C18Im at 100 °C. (d) Proposed model of the liquid crystal structures. POSS, polyhedral oligomeric silsesquioxane.
Conclusion
POSS-based imidazolium salts with various alkyl chain lengths possessed melting temperatures below 100 °C and turned out to be ILs. For butyl to octadecyl alkyl chains, the POSS ion salts showed higher thermal stabilities and lower melting temperatures than the corresponding Arm ion salts. The properties of the POSS-C18Im samples can be explained by considering the rigid cubic structure and the molecular shape of the POSS core. During the melting process, the POSS core reduces molecular interactions between the ion pairs. After melting, the POSS core induces regular structures in the ion salts, even in the liquid phase. Thus, it was demonstrated that POSS-C18Im samples showed much higher mesophase stabilities. Thermally stable thermotropic IL crystals are useful in modern electronic devices. These findings should have important implications for the preparation of the new series of IL crystals involving nanostructures.

Chemical structures and synthetic scheme for preparing the ion salts used in this study.
References
Binnemans, K. Ionic liquid crystals. Chem. Rev. 105, 4148–4204 (2005).
Axenov, K. & Laschat, S. Thermotropic ionic liquid crystals. Materials 4, 206–259 (2011).
Goh, M., Park, J., Han, Y., Ahn, S. & Akagi, K. Chirality transfer from atropisomeric chiral inducers to nematic and smectic liquid crystals – synthesis and characterization of di- and tetra-substituted axially chiral binaphthyl derivatives. J. Mater. Chem. 22, 25011–25018 (2012).
Wezenberg, S. J., Ferroni, F., Pieraccini, S., Schweizer, W. B., Ferrarini, A., Spada, G. P. & Diederich, F. Effective cholesteric liquid crystal inducers based on axially chiral alleno-acetylenes. RSC Adv. 3, 22845–22848 (2013).
Yoshio, M., Mukai, T., Ohno, H. & Kato, T. One-dimensional ion transport in self-organized columnar ionic liquids. J. Am. Chem. Soc. 126, 994–995 (2004).
Yoshio, M., Kagata, T., Hoshino, K., Mukai, T., Ohno, H. & Kato, T. One-dimensional ion-conductive polymer films: alignment and fixation of ionic channels formed by self-organization of polymerizable columnar liquid crystals. J. Am. Chem. Soc. 128, 5570–5577 (2006).
Kohler, F. T.U., Morain, B., Weiss, A., Laurin, M., Libuda, J., Wagner, V., Melcher, B. U., Wang, X., Meyer, K. & Wasserscheid, P. Surface-functionalized ionic liquid crystal-supported ionic liquid phase materials: ionic liquid crystals in mesopores. ChemPhysChem 12, 3539–3546 (2011).
Shi, F., Zhang, Q., Li, D. & Deng, Y. Silica-gel-confined ionic liquids: a new attempt for the development of supported nanoliquid catalysis. Chem. Eur. J. 12, 5279–5288 (2005).
Carmichael, A. J., Hardacre, C., Holbrey, J. D., Nieuwenhuyzen, M. & Seddon, K. R. Molecular layering and local order in thin films of 1-alkyl-3-methylimidazolium ionic liquids using X-ray reflectivity. Mol. Phys. 99, 795–800 (2001).
Tanaka, K. & Chujo, Y. Chemicals-inspired biomaterials; developing biomaterials inspired by material science based on POSS. Bull. Chem. Soc. Jpn 86, 1231–1239 (2013).
Kaneko, Y., Toyodome, H., Mizumo, T., Shikinaka, K. & Iyi, N. Preparation of a sulfo-group-containing rod-like polysilsesquioxane with a hexagonally stacked structure and its proton conductivity. Chem. Eur. J. 20, 9394–9399 (2014).
Toyodome, H., Higo, Y., Sasai, R., Kurawaki, J. & Kaneko, Y. Behavior of chiral induction from polysilsesquioxanes bearing chiral and ammonium groups to anionic pyrene derivatives. J. Nanosci. Nanotechnol. 13, 3074–3078 (2013).
Yamamoto, E., Kitahara, M., Tsumura, T. & Kuroda, K. Preparation of size-controlled monodisperse colloidal mesoporous silica nanoparticles and fabrication of colloidal crystals. Chem. Mater. 26, 2927–2933 (2014).
Nakato, T., Yamashita, Y., Mouria, E. & Kuroda, K. Multiphase coexistence and destabilization of liquid crystalline binary nanosheet colloids of titanate and clay. Soft Mater. 10, 3161–3165 (2014).
Kuroda, K., Shimojima, A., Kawahara, K., Wakabayashi, R., Tamura, Y., Asakura, Y. & Kitahara, M. Utilization of alkoxysilyl groups for the creation of structurally controlled siloxane-based nanomaterials. Chem. Mater. 26, 211–220 (2014).
Jovanovski, V., Orel, B., Ješe, R., Vuk, A.Š., Mali, G., Hočevar, S. B., Grdadolnik, J., Stathatos, E. & Lianos, P. Novel polysilsesquioxane−I-/I3- ionic electrolyte for dye-sensitized photoelectrochemical cells. J. Phys. Chem. B 109, 14387–14395 (2005).
Jovanovski, V., Orel, B., Ješe, R., Mali, G., Stathatos, E. & Lianos, P. Degradation of aqueous methyl tert-butyl ether by photochemical, biological, and their combined processes. Int. J. Photoenergy 8, 1–8 (2006).
Vuk, A.Š., Jovanovski, V., Pollet-Villard, A., Jerman, I. & Orel, B. Imidazolium-based ionic liquid derivatives for application in electrochromatic devices. Sol. Energy Mater. Sol. Cells 92, 126–135 (2008).
Subianto, S., Mistry, M. K., Choudhury, N. R., Dutta, N. K. & Knott, R. Composite polymer electrolyte containing ionic liquid and functionalized polyhedral oligomeric silsesquioxanes for anhydrous PEM applications. ACS Appl. Mater. Interfaces 6, 1173–1182 (2009).
Naka, K., Shinke, R., Yamada, M., Djouadi Belkada, F., Aijo, Y., Irie, Y., Ram Shankar, S., Sai Smaran, K., Matsumi, N., Tomita, S. & Sakurai, S. Synthesis of imidazolium salt-terminated poly(amidoamine)-typed POSS-core dendrimers and their solution and bulk properties. Polym. J. 46, 42–51 (2014).
Naka, K., Masuoka, S., Shinke, R. & Yamada, M. Synthesis of first and second generation imidazole-terminated POSS-core dendrimers and their pH responsive and coordination properties. Polym. J. 44, 353–359 (2012).
Ishii, T., Mizumo, T. & Kaneko, Y. Facile preparation of ionic liquid containing silsesquioxane framework. Bull. Chem. Soc. Jpn 87, 155–159 (2014).
Tanaka, K. & Chujo, Y. Unique properties of amphiphilic POSS and their applications. Polym. J. 45, 247–254 (2013).
Tanaka, K. & Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 22, 1733–1746 (2012).
Tanaka, K., Ishiguro, F. & Chujo, Y. POSS ionic liquid. J. Am. Chem. Soc. 132, 17649–17651 (2010).
Tanaka, K., Ishiguro, F. & Chujo, Y. Thermodynamic study of POSS-based ionic liquids with various numbers of ion pairs. Polym. J. 43, 708–713 (2011).
Jeon, J.-H., Tanaka, K. & Chujo, Y. POSS fillers for modulating thermal properties of ionic liquids. RSC Adv. 3, 2422–2427 (2013).
Jeon, J.-H., Tanaka, K. & Chujo, Y. Synthesis of sulfonic acid-containing POSS and its filler effects for enhancing thermal stabilities and lowering melting temperatures of ionic liquids. J. Mater. Chem. A 2, 624–630 (2014).
Rogers, R. D. & Seddon, K. R. Ionic liquids—solvents of the future? Science 302, 792–793 (2003).
Holbrey, J. D. & Seddon, K. R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Chem. Soc. Dalton Trans. 2133–2139 (1999).
Acknowledgements
We thank Professor Susumu Kitagawa and Dr Satoshi Horike (Kyoto University) for the use of the VT-XRD instrument and Professor Hirokazu Hasegawa and Dr Kenji Saijo (Kyoto University) for the use of the SAXS instrument. We acknowledge Professor Kazuo Akagi and Dr Munju Goh (Kyoto University) for their advice concerning the structural analysis of the liquid crystals. This work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘New Polymeric Materials Based on Element-Blocks (No.2401)’ (25102521) from The Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the NPG Asia Materials website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tanaka, K., Ishiguro, F., Jeon, JH. et al. POSS ionic liquid crystals. NPG Asia Mater 7, e174 (2015). https://doi.org/10.1038/am.2015.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/am.2015.28
This article is cited by
-
Recent progress in the development of advanced element-block materials
Polymer Journal (2018)
-
A novel room temperature POSS ionic liquid-based solid polymer electrolyte
Journal of Materials Science (2018)
-
Remarkably high miscibility of octa-substituted POSS with commodity conjugated polymers and molecular fillers for the improvement of homogeneities of polymer matrices
Polymer Journal (2016)
-
Preparation of photo-responsive hybrid materials based on hydrogels involving imidazolium-presenting gold nanoparticles
Polymer Journal (2016)