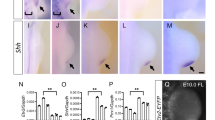
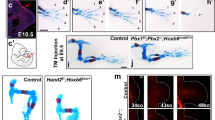
Abstract
The Drosophila homeobox gene extradenticle (exd) encodes a highly conserved cofactor of Hox proteins1,2,3. exd activity is regulated post-translationally by a mechanism involving nuclear translocation4,5; only nuclear Exd protein is functional. The exd gene is required for patterning of the proximal region of the leg6,7, whereas patterning of the distal region requires signalling by the Wingless (Wg) and Decapentaplegic (Dpp)8,9 proteins, which are in turn activated by Hedgehog (Hh)10. Here we show that exd function and Dpp/Wg signalling are antagonistic and divide the leg into two mutually exclusive domains. In the proximal domain, exd activity prevents cells from responding to Dpp and Wg. Conversely, in the distal domain, exd function is suppressed by the Dpp/Wg response gene Distal-less (Dll), which prevents the nuclear transport of Exd. We also found that the product of a murine homologue of exd (Pbx1) is regulated at the subcellular level, and that its pattern of nuclear localization in the mouse limb resembles that of Exd in the Drosophila leg. These findings suggest that the division of the limb into two antagonistic domains, as defined by exd (Pbx1) function and Hh signalling, may be a general feature of limb development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peifer, M. & Wieschaus, E. Mutations in the Drosophila gene extradenticle affect the way specific homeo domain proteins regulate segmental identity. Genes Dev. 4, 1209–1223 (1990).
Rauskolb, C., Peifer, M. & Wieschaus, E. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx-1. Cell 74, 1101–1112 (1993).
Mann, R. & Chan, S. K. Extra specificity from extradenticle: the partnership between Hox and exd/Pbx homeodomain proteins. Trends Genet. 12, 258–262 (1996).
Mann, R. & Abu-Shaar, M. Nuclear import of the homeodomain protein Extradenticle in response to Wg and Dpp signalling. Nature 383, 630–633 (1996).
Aspland, S. E. & White, R. A. H. Nucleocytoplasmic localization of extradenticle protein is spatially regulated throughout development in Drosophila. Development 124, 741–747 (1997).
González-Crespo, S. & Morata, G. Control of Drosophila adult pattern by extradenticle. Development 121, 2117–2125 (1995).
Rauskolb, C., Smith, K. M., Peifer, M. & Wieschaus, E. extradenticle determines segmental identities throughout Drosophila development. Development 121, 3663–3673 (1995).
Diaz-Benjumea, F. J., Cohen, B. & Cohen, S. M. Cell interaction between compartments establishes the proximal–distal axis of Drosophila legs. Nature 372, 175–179 (1994).
Lecuit, T. & Cohen, S. M. Proximal–distal axis formation in the Drosophila leg. Nature 388, 139–145 (1997).
Basler, K. & Struhl, G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368, 208–214 (1994).
Cohen, S. M. & Jürgens, G. Proximo-distal pattern formation in Drosophila: cell autonomous requirements for Distal-less gene activity in limb development. EMBO J. 8, 2045–2055 (1989).
Cohen, B., Simcox, A. A. & Cohen, S. M. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 117, 597–608 (1993).
Goto, S. & Hayashi, S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development 124, 125–132 (1997).
Mardon, G., Solomon, N. M. & Rubin, G. M. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473–3486 (1994).
Grim, S. & Pflugfelder, G. O. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science 271, 1601–1604 (1996).
González-Crespo, S. & Morata, G. Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development 122, 3921–3928 (1996).
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996).
Monica, K., Galili, N., Nourse, J., Saltman, D. & Cleary, M. L. PBX2 and PBX3, new homeobox genes with extensive homology ot the human protooncogene PBX1. Mol. Cell. Biol. 11, 6149–6157 (1991).
Brook, W. J. & Cohen, S. M. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science 273, 1373–1377 (1996).
Nellen, D., Burke, R., Struhl, G. & Basler, K. Direct and long-range action of a Dpp morphogen gradient. Cell 85, 357–368 (1996).
Rieckohf, G., Casares, F., Ryoo, H. D., Abu-Shaar, M. & Mann, R. Nuclear translocation of Extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171–183 (1997).
Penton, A. & Hoffman, F. M. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature 382, 162–165 (1996).
Jiang, J. & Struhl, G. Complementary and mutually exclusive activities of Decapentaplegic and Wingless organize axial patterning during Drosophila leg development. Cell 86, 401–409 (1996).
Cohen, S. M., Brönner, G., Küntter, F., Jürgens, G. & Jäckle, H. Distal-less encodes a homeodomain protein required for limb development in Drosophila. Nature 338, 432–434 (1989).
Morata, G. & Ripoll, P. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975).
Zecca, M., Basler, K. & Struhl, G. Direct and long range action of a wingless morphogen gradient. Cell 87, 833–844 (1996).
Fuse, N., Hirose, S. & Hayashi, S. Determination of wing cell fate by the escargot and snail genes in Drosophila. Development 122, 1059–1067 (1996).
Torres, M., Gómez-Pardo, E., Dressler, G. R. & Gruss, P. Pax2 controls multiple steps of urogenital development. Development 121, 4057–4065 (1995).
Vachon, G. et al. Homeotic genes of the bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell 71, 437–450 (1992).
Acknowledgements
We thank K. Basler, S. Carroll, S. Cohen, G. Mardon, G. Pflugfelder and G. Struhl for fly stocks and antibodies; F. Casares, E. Sánchez-Herrero and I. Guerrero for comments on the manuscript; J. Casanova for critical help; and A. Bosch for help with the confocal microscope. This work was supported by the Dirección General de Investigación Científica y Técnica (G.M.), a Human Frontiers grant (G.M. and R.S.M.), an institutional grant of the Fundación Ramón Areces to the Centro de Biología Molecular, Institutional grants of the CSIC and Pharmacia-Upjohn to the Departamento de Immunologia y Oncologia, and an NIH grant to R.S.M., who is a scholar of the Leukemia Society of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Crespo, S., Abu-Shaar, M., Torres, M. et al. Antagonism between extradenticle function and Hedgehog signalling in the developing limb. Nature 394, 196–200 (1998). https://doi.org/10.1038/28197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/28197
This article is cited by
-
Transcription factor paralogs orchestrate alternative gene regulatory networks by context-dependent cooperation with multiple cofactors
Nature Communications (2022)
-
Appendage patterning in the primitively wingless hexapods Thermobia domestica (Zygentoma: Lepismatidae) and Folsomia candida (Collembola: Isotomidae)
Development Genes and Evolution (2013)
-
A potential role for the homeoprotein Hhex in hepatocellular carcinoma progression
Medical Oncology (2012)
-
Beinentwicklung und Gliedmaßen-Evolution bei Spinnen
BIOspektrum (2012)
-
Chromosomal binding sites of the homeotic cofactor Homothorax
Molecular Genetics and Genomics (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.