Abstract
A yeast artificial chromosome (YAC) contig was constructed encompassing the entire region on chromosome 17p13 where the autosomal recessive disorder infantile nephropathic cystinosis (MIM 21980, CTNS-LSB) has been genetically mapped. It comprises seven clones ordered by their content of a series of six sequence-tagged sites (STSs). Fluorescence in situ hybridisation (FISH) revealed two chimaeric clones. The order of four polymorphic STSs mapped with the contig was consistent with that of the known genetic map with the exception of markers D17S1583 (AFMb307zg5) and D17S1798 (AFMa202xf5) where a telomeric location of D17S1583 was inferred from the contig; two non-polymorphic STSs were localised within the marker framework. From the analysis of recombination events in an unaffected individual as defined by leucocyte cystine levels we support the high-resolution mapping of this region to a small genetic interval and show that it is entirely represented on a single, non-chimaeric YAC clone in the contig.
Similar content being viewed by others
Introduction
The construction of detailed physical maps of the human genome is an important step in the identification and cloning of disease genes. Recently, the recessive disorder infantile nephropathic cystinosis (MIM 219800, CTNS-LSB) has been genetically mapped to a region on 17p, defined by the polymorphic markers D17S1583 and D17S796 [1]. Further attempts to define the cystinosis region have been made and it is now believed, on the basis of critical recombination events in heterozygous gene carriers, to reside on a small interval flanked by the markers D17S1798 and D17S1828 [2].
In the course of our search for the cystinosis gene, we had already excluded the disease from a large part of the entire human genome when the mapping to chromosome 17p was reported. We confirmed linkage to microsatellite markers for chromosome 17p in 19 German pedigrees with infantile nephropathic cystinosis and analysed critical recombination events to shorten the genetic interval of the critical region; this was facilitated by discriminating asymptomatic heterozygous individuals by measuring their cystine content in leucocytes and thus increasing informativity in two-generation, single-affected pedigrees. We then constructed a continuous array of overlapping yeast artificial chromosome (YAC) clones as a further step towards physical mapping and identification of the gene responsible for cystinosis.
Methods
Subjects
Eighteen two-generation pedigrees, among them 5 multiplex pedigrees with 2 affected offspring, were included in the study and comprised a total of 53 meioses, 23 of them were affected individuals. With the exception of pedigree MS0096, which is of Turkish descent, the families originate from Germany and Switzerland; there was no known history of consanguinity in any of the families. Affected individuals presented with typical infantile nephropathic cystinosis during early childhood. Diagnosis of cystinosis was established by measuring the cystine content of PMNL from heparinised blood [3, 4] by cation exchange column chromatography and ninhydrine detection using an amino acid analyser (Biotronik LC5000) [5].
Microsatellite Genotyping
Primer sequences and PCR conditions were as reported [6–8]; markers were typed using 5′-digoxigenin-labelled forward primers, separated by direct blotting electrophoresis (GATC, MWG Biotech) and visualised as described [9].
Linkage Analysis
Two-point lod scores for linkage of the disease locus and a genetic marker were calculated using MLINK from the LINKAGE (version 5.1) package of programs [10]. The disease frequency was set to 0.001, and marker allele frequencies were assumed to be equal for all alleles observed for a given marker. A penetrance of 1.0 was assumed for affected individuals, all unaffected individuals were treated equally, i.e. heterozygote status was not assigned in linkage analysis.
Detection of STSs in YAC Pools and Isolated YAC DNA
YAC pools from the ICI [11] and ICRF [12] libraries obtained through the UK HGMP Resource Centre, Hinxton, UK, and the pooled CEPH mega-YAC library [13] provided by the Fondation Jean Dausset CEPH, Paris, France, were screened using standard PCR protocols with the same primers as above. Sequences for the markers D17S2054 (WI7337) and D17S2027 (WI9827) were obtained from the Genome Database. For the detection of STSs DNA isolated from single YAC clones, the same PCR conditions using 5′-digoxigenin-labelled primers were used. Products were separated by direct blotting electrophoresis and detected as above.
FISH Mapping
For FISH mapping, YAC DNA was amplified by Alu-PCR using the primers CL1 and CL2 described in the literature [14]. In a total volume of 50 µl containing approximately 100 ng YAC DNA, 0.25 µM of each primer, 75 m M Tris-HCl, pH 9.0, 20 mM (NH4)2SO4, 0.01% Tween 20, 1.5 mM MgCl2, 250 µM each of dNTPs, and 0.5 units of Taq DNA polymerase (GoldStar, Eurogentec) 35 PCR cycles were carried out with 96 °C for 1 min, 37 °C for 30 s, and 72 ° C for 6 min. During the first cycle, denaturation was prolonged to 4 min, and in the last cycle primer extension was lengthened to 10 min. The PCR products were purified via QIAquick PCR purification columns (Qiagen) and 1 µg of the resulting DNA was labelled with Biotin-14-dATP using the BioNick kit (BRL). Chromosome preparations from phytohaemagglutinin (PHA)-stimulated normal human lymphocytes were obtained using standard procedures. In situ hybridisation, post-hybridisation washes and probe detection were performed as described previously [15] with minor modifications: 250–300 ng of the biotinylated YAC-DNA was preannealed with 10 µg Cot 1 DNA (BRL) and hybridisation was carried out overnight. Probe detection with avidin-FITC (Vector Laboratories, diluted 1:500 in 4 × SSC, 0.05% Tween 20, pH 7.0, 5% low-fat dried milk) was done with one round of signal amplification. Slides were mounted with Vectashield (Vector Laboratories) containing 0.1 µg/ml DAPI as counterstain. Probe signals were analysed with a Zeiss Axioscop microscope equipped with a Pinkel No. 1 fluorescence filter set. Digital grey-scale images of probe signals and DAPI-stained chromosomes were captured separately with a cooled CCD camera (KAF1400, Photometrics) and merged using IPlab Spectrum software (Signal Analytics, Vienna, Va., USA).
Results
Genetic Map and Fine-Mapping of the Cystinosis Locus
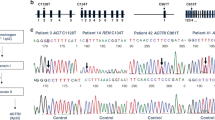
The polymorphic microsatellite repeat markers D17S1583 (AFMb307zg5), D17S1798 (AFMa202xf5), D17S1828 (AFMb013zbl), D17S1584 (AFMa061za9), D17S938 (AFM263wh5), and D17S796 (AFM177xh6) were used to type a total of 18 pedigrees with 23 children affected with infantile nephropathic cystinosis. Segregation analysis, based on the known genetic order of the markers, shows one critical recombination event in an unaffected at-risk sib in pedigree MS0096 who is not heterozygous on the basis of PMNL cystine content (207 in fig. 1A); the affected child (204 in fig. 1A) is homozygousfor markers D17S1798, D17S1828, D17S1584 and D17S938. D17S1798 is not informative in the pedigree, nor is D17S1583. In pedigree MS0131, a cross-over between D17S1583 and D17S1828 in the unaffected child places D17S1583 towards the telomere from the cystinosis locus (fig. 1B); D17S1583 and D17S1798 cannot be separated by recombination in any of the pedigrees analysed. As shown in table 1, two-point lod scores above +3.0 were obtained with several markers from the expected region between D17S1583 and D17S1584. D17S1798 is not fully informative in all the pedigrees. Note that these results are obtained without taking into account polymorphonuclear leucocyte (PMNL) cystine values and, therefore, heterozygous carriers and non-carriers are not differentiated in the calculations. D17S1583 is excluded from the cystinosis locus by recombination in an affected individual and thus the lod score is −∞, the recombination excluding D17S1828 from the cystinosis region, however, is observed in an unaffected at-risk individual and therefore is not reflected in the results of linkage analysis.
Pedigrees MS0096 and MS0131 showing critical recombination events placing the cystinosis in a region flanked by D17S1583 and D17S1828. In both pedigrees, filled symbols are used for affected individuals and open symbols denote unaffected individuals; in pedigree MS0096 (A), cystine content of PMNL is shown in micromoles per gram PMNL protein and heterozygous at-risk individuals as defined by a PMNL cystine content above the detection level of 0.01 µmol/g protein are denoted by dots within open symbols. Genotypes for the markers D17S1583, D17S1798, D17S1828, D17S1584, D17S938, and D17S796 are shown (top to bottom); those alleles where phase could not be established are shown in brackets. A cross-over, indicated by an arrow, occurs in the paternal haplotype of individual 207 who is at-risk but not heterozygous; this suggests the cystinosis locus to be located distal to D17S1828. D17S1798 is not informative in the individual showing recombination and thus cannot be excluded from the cystinosis region. In pedigree MS0131 (B), the unaffected and the affected child share the same alleles of D17S1583 which is therefore excluded from the cystinosis locus (arrow); D17S1798 is not informative. Note that the affected individual 204 in pedigree MS0096 is homozygous for all markers from D17S1798 to D17S938 (for details on pedigrees, marker genotyping, and cystine measurements refer to the Methods section).
Identification of YAC Clones Mapping to the Cystinosis Region by STS Screening of Pooled Libraries and Database Searching, Cytogenetic Localisation of YAC Clones, and Determination of YAC Chimaerism by FISH
A search in the CEPH/Généthon integrated database revealed that two YAC clones had been reported to contain at least one of the STSs representing each of the five polymorphic microsatellite repeat markers used for linkage analysis. YAC 767_f_9 was reported positive for D17S1828 as well as for the non-polymorphic marker D17S2054 (WI7337), and YAC clone 868_h_8 for D17S1798 and D17S2027 (WI9827). D17S2054 (WI7337) was also reported to be present in YAC 926_e_5 which we, too, identified as a positive clone in our YAC pool screening using the four polymorphic as well as the two non-polymorphic STSs, along with YACs 782_b_1 and 926_e_5 from the CEPH mega-YAC library [13] and 4X54-A9 (y900A0954) and 4X98-D4 (y900D0498) from the ICRF YAC library [12] for which no STSs had been reported previously (table 2). No positive screening results were obtained in the ICI YAC library with any of the STSs used [11]. FISH mapping was performed to determine the chromosomal content of the YAC clones and the results are also shown in table 2. Four clones, ICRF 4X54-A9 and CEPH YACs 767_f_9, 926_e_5, and 756_b_9, were found to map exclusively to chromosome 17p13 (fig. 2); the remaining clones were found chimaeric giving signals on various chromosomes and chromosomal regions (table 2).
YAC Contig Construction by STS Content and Integration of Genetic and Physical Mapping Data
Table 2 summarises our data on the STS content determined in the 7 YAC clones that had been identified by database search and pool screening as containing at least one of the genetic markers for the cystinosis region. Five clones connect at least two STSs, i.e. they are level 1 resolution. The contig confirms the formerly reported order of loci on the genetic map with the exception of two loci, D17S1798 and D17S1583, discussed below; it also allows to place markers relative to each other that had not been resolved by recombination events (fig. 3). The non-polymorphic STS WI9887 is present in YAC 4X54-A9; this clone also contains D17S1798 (AFMa202xf5) which, in YAC clones 4X98-D4 and 767_f_9, segregates with the more centromeric marker D17S1828 (AFMb013zbl). YAC 868_h_9 shows segregation of both D17S1798 and D17S2027 (WI9827) with the marker D17S1583, placing D17S2027 (WI9827) between D17S1583 and D17S1798. These findings suggest the physical order of these two polymorphic markers with D17S1583 telomeric from D17S2027 (WI9827) and therefore D17S1798, too. D17S2054 (WI7337) is contained in YAC clone 926_e_5 together with D17S1828; in YAC 767_f_9 this STS segregates with D17S1798 and D17S1828 as well as with D17S1584 which is located towards the centromere from D17S1828; D17S2054 (WI7337) is thus most likely situated between these two markers. This physical location is consistent with the finding that YAC 756_b_9 contains D17S15 84 as the only one of the four STSs segregating on 767_f_9.
Integrated genetic and physical map and Y AC contig comprising the cystinosis critical region. Shown on top (A) is the known genetic order, from telomere (left) to centromere (right) of the markers D17S1583, D17S1798, D17S1828, and D17S1584 and the refined genetic order as derived from recombination events observed in this study (8). Note that the order of D17S1583 and D17S1798 has been retained as reported in Jean et al. [2] and thus differs from the recently published genetic map [8]. Y AC clones are positioned according to their STS content as indicated by hatched symbols with squares for the polymorphic STSs contained in the genetic map and round symbols for the non-polymorphic markers D 17S2027 (WI9827) and D17S2054 (WI7337). The position of these non-polymorphic markers within the framework of genetic markers is indicated in C, which represents the integrated genetic and physical map of the region. The hatched area shows where the cystinosis locus is situated on the basis of recombination events reported in the literature[1, 2] and in this study.
Discussion
Infantile nephropathic cystinosis has been mapped to chromosome 17p within the interval defined by markers D17S1583 and D17S796 [1]. In a recent report, high-resolution mapping using combined biochemical and genotyping data suggested a critical region flanked by markers D17S1798 and D17S1828 [2]. In our own study, we have used a similar approach and identified at-risk individuals as either heterozygous or unaffected by determining cystine content in PMNL of obligate heterozygotes and at-risk sibs. In an at-risk individual defined as non-heterozygous, we observed a critical recombination event that places the disease locus distal to D17S1828. This confirms the reported fine-mapping of the cystinosis locus where determination of the centromeric border of the region was based on only a single recombination observed in a heterozygous at-risk individual [2]. In our sample, because D17S1798 is not informative in those pedigrees with recombinations in the cystinosis region, the closest flanking marker towards the telomere is D17S1583 as in the initial report on linkage [1]. From their segregation data the authors of the French study [2] suggest that D17S1798, rather than D17S1583, forms the telomeric border of the cystinosis region; interestingly and similar to these data, we observed a region of homozygosity across the entire region flanked by D17S1583 and D17S796 in the affected child in the one Turkish pedigree in our study although no history of consanguinity is known. In summary, our linkage and segregation data corroborate the use of biochemical data for heterozygote detection for disease mapping; they also confirm the genetic fine-mapping data and thus help to narrow the cystinosis region to a very small genetic interval on chromosome 17p13 defined by the markers D17S1798 and D17S1828.
By STS screening of the ICI, ICRF and CEPH YAC libraries, we were able to identify YAC clones from this region. A continuous array of seven YAC clones ordered by their STS content spans the entire cystinosis region. FISH mapping localises the clones to chromosome 17p13 although, as was to be expected, it shows several of the YAC clones to be chimeric. Even chimeric clones, however, can still be used for the physical mapping of STSs and thus to integrate genetic and physical mapping information. The non-polymorphic STSs D17S2027 (WI9827) and D17S2054 (WI7337) have been placed in the marker framework where their respective positions could be inferred from the STSs content of the YACs in the contig. There is one apparent difference between the genetic marker order as reported in the most recent update of the genetic map [8] and the relative positions of D17S1583 (AFMb307zg5, reassigned D17S1845) and D17S1798 (AFMa202xf5). Our physical mapping data, obtained by determining the STS content of YAC clones as shown in table 2 and summarised in figure 3, however, suggest an inferred physical order of these two markers, with D17S1583 located telomeric with respect to D17S1798, which is in agreement with the genetic order assumed in the reported linkage studies [2].
Two YAC clones in the contig, 4X98-D4 (y900D0498) (ICRF) and 767_f_9 (CEPH) contain both markers, D17S1798 and D17S1828, flanking the refined cystinosis critical region as it has been reported [1, 2] and as is supported by our own linkage and segregation data. One of them, 767_f_9, is a non-chimaeric clone; its availability should help to identify and eventually clone the gene responsible for infantile nephropathic cystinosis.
References
McDowell GA, Gahl WA, Stephenson LA, Schneider JA, Weissenbach J, Polymeropoulos MH, Town MM, van’t Hoff W, Farrall M, Mathew CG: Linkage of the gene for cystinosis to markers on the short arm of chromosome 17. Nature Genet 1995;10:246–248.
Jean G, Fuchshuber A, Town MM, Gribouval O, Schneider JA, Broyer M, van’t Hoff W, Niaudet P, Antignac C: High-resolution mapping of the gene for cystinosis, using combined biochemical and linkage analysis. Am J Hum Genet 1996;58:535–543.
Smolin LA, Clark KF, Schneider JA: An improved method for heterozygote detection of cystinosis, using polymorphonuclear leukocytes. Am J Hum Genet 1987;41:266–275.
Schneider JA, Bradley K, Seegmiller JE: Increased cystine in leukocytes from individuals homozygous and heterozygous for cystinosis. Science 1967;157:1321–1322.
Lee PL: Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med 1974; 10:107–121.
Weissenbach J, Gyapay G, Dib C, Vignal A, Morissette J, Millasseau P, Vaysseix G, Lathrop M: A second-generation linkage map of the human genome. Nature 1992;359:794–801.
Gyapay G, Morissette J, Vignal A, Dib C, Fizames C, Millasseau P, Marc S, Bernardi G, Lathrop M, Weissenbach J: The 1993–94 Généthon human genetic linkage map. Nature Genet 1994;7:246–339.
Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J: A comprehensive genetic map of the human genome based on 5264 microsatellites. Nature 1996;380:152–154.
Mekus F, Dörk T, Deufel T, Morral N, Tümmler B: Analysis of microsatellites by direct blotting electrophoresis and chemoluminescence detection. Electrophoresis 1995;16:1886–1888.
Lathrop GM, Lalouel J-M: Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 1984;36:460.
Anand R, Riley JH, Butler R, Smith JC, Markham AF: A 3.5 genome equivalent multiaccess YAC library — Construction, characterization, screening and storage. Nucleic Acids Res 1990; 18:1951–1956.
Larin Z, Monaco AP, Lehrach H: Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci USA 1991;88:4123–4127.
Chumakov I, Rigault P, Guillou S, Ougen P, Bil-laut A, Guasconi G, Gervy P, LeGall I, Soularue P, Grinas L, Bougueleret L, Bellanné-Chantelot C, Lacroix B, Barillot E, Gesnouin P, Pock S, Vaysseix G, Frelat G, Schmitz A, Sambucy JL, Bosch A, Estivill X, Weissenbach J, Vignal A, Riethmann H, Cox D, Patterson D, Gardiner K, Hattori M, Sakaki Y, Ichikawa H, Ohki M, LePaslier D, Heilig R, Antonarakis SE, Cohen D: Continuum of overlapping clones spanning the entire human chromosome 21q. Nature 1992;359:380–387.
Lengauer C, Green ED, Cremer T: Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics 1992; 13:826–828.
Senger G, Ragoussis J, Trowsdale J, Sheer D: Fine mapping of the human MHC class II region within chromosome band 6p21 and evaluation of probe ordering using interphase fluorescence in situ hybridization. Cytogenet Cell Genet 1993;64:49–53.
Acknowledgements
We gratefully acknowledge the valuable work of the UK Human Genome Mapping Project Resource Centre, Hinxton, UK, and the Fondation Jean Dausset CEPH, Paris, which has provided YAC pools and YAC clones. Part of this work was supported by a DFG grant (Ha 756/8-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, U., Senger, G., Rählmann, M. et al. Nephropathic Cystinosis (CTNS-LSB): Construction of a YAC Contig Comprising the Refined Critical Region on Chromosome 17p13. Eur J Hum Genet 5, 9–14 (1997). https://doi.org/10.1007/BF03405871
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03405871