Abstract
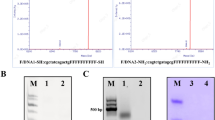
The three-component nanoparticle of this investigation consisted of an anti-type I regulatory subunit α of the cyclic AMP-dependent protein kinase A (RIα) antisense phosphorodiamidate morpholino (MORF) oligomer, a tat peptide and the anti-HER2 Herceptin antibody each biotinylated and each linked via streptavidin and tested in SUM190 (HER2+), SUM149 (HER2−) and SK-BR-3 (HER2+) cells in culture, using both radioactivity and fluorescent labels on the antisense and control sense MORF. Within the nanoparticle, the antibody provides specific binding to the target cells, the tat improves cellular delivery and the MORF provides the specific retention of the radioactivity in the target cell nucleus. The results show that within the nanoparticle, the Herceptin was still able to bind to its determinant; that the MORF escaped entrapment with its mRNA-binding ability preserved and that the tat maintained its carrier function. Fluorescence microscopy showed evidence of antisense MORF internalization, separation from Herceptin and migration to the nucleus. In conclusion, streptavidin appears to provide an easy means of mixing and matching components to improve the tumor-specific targeting, cell membrane transport, pharmacokinetics and other properties of antisense and other oligomers. Combining the three components of this investigation with streptavidin apparently did not interfere with the properties of each component in cell culture and significantly improved delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hnatowich DJ, Nakamura K . The influence of chemical structure of DNA and other oligomer radiopharmaceuticals on tumor delivery. Curr Opin Mol Ther 2006; 8: 136–143.
Nakamura K, Wang Y, Liu X, Kubo A, Hnatowich DJ . Addition of TAT, polyarginine and cholesterol carriers to MDR1 antisense DNA using streptavidin as linker. J Nucl Med 2006; 47 (Suppl): 512. Abstract.
Chu TC, Twu KY, Ellington AD, Levy M . Aptamer mediated siRNA delivery. Nucleic Acids Res 2006; 34: e73.
Wang Y, Nakamura K, Liu X, Kitamura N, Kubo A, Hnatowich DJ . Simplified preparation via streptavidin of antisense oligomers/carriers nanoparticles showing improved cellular delivery in culture. Bioconjug Chem 2007; 18: 1338–1343.
Nakamura K, Wang Y, Liu X, Kubo N, Hnatowich DJ . Cell culture and xengrafted animal studies of radiolabeled antisense DNA/carrier constructs using streptavidin as linker. J Nucl Med 2007; 48: 1845–1852.
Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, Symmans WF et al. Targeted therapy in breast cancer: the HER-2/neu gene and protein. Mol Cell Proteomics 2004; 3: 379–398.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182.
Albarran B, To R, Stayton PS . A TAT-streptavidin fusion protein directs uptake of biotinylated cargo into mammalian cells. Protein Eng Des Sel 2005; 18: 147–152.
Rinne J, Albarran B, Jylhävä J, Ihalainen TO, Kankaanpää P, Hytönen VP et al. Internalization of novel non-viral vector TAT-streptavidin into human cells. BMC Biotechnol 2007; 7: 1.
Wang Y, Liu X, Hnatowich DJ . An improved synthesis of NHS-MAG3 for conjugation and radiolabeling of biomolecules with (99m)Tc at room temperature. Nat Protoc 2007; 2: 972–978.
Bussolati G, Montemurro F, Righi L, Donadio M, Aglietta M, Sapino A . A modified Trastuzumab antibody for the immunohistochemical detection of HER-2 overexpression in breast cancer. Br J Cancer 2005; 92: 1261–1267.
Zhang YM, Wang Y, Liu N, Zhu ZH, Rusckowski M, Hnatowich DJ . In vitro investigations of tumor targeting with 99mTc–labeled antisense DNA. J Nucl Med 2001; 42: 1660–1669.
Behlke MA . Progress towards in vivo use of siRNAs. Mol Ther 2006; 13: 644–670.
Goldenberg DM, Sharkey RM, Barbet J, Chatal J-F . Radioactive antibodies: selective targeting and treatment of cancer and other diseases. Appl Radiol 2007; 36: 10–29.
Tada H, Higuchi H, Wanatabe TM, Ohuchi N . In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res 2007; 67: 1138–1144.
Liu Y, Miyoshi H, Nakamura M . Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer 2007; 120: 2527–2537.
Mirochnik Y, Rubenstein M, Guinan P . Targeting of biotinylated oligonucleotides to prostate tumors with antibody-based delivery vehicles. J Drug Target 2007; 15: 342–350.
Zhang YM, Liu CB, Liu N, Ferro Flores G, He J, Rusckowski M et al. Electrostatic binding with tat and other cationic peptides increases cell accumulation of 99mTc-antisense DNAs without entrapment. Mol Imaging Biol 2003; 5: 240–247.
Liu X, Nakamura K, Wang Y, Wang Y, Liu G, He J et al. Initial mechanistic studies of antisense targeting in cells. J Nucl Med 2006; 47: 360–368.
Buchegger F, Perillo-Adamer F, Dupertuis YM, Delaloye AB . Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging 2006; 33: 1352–1363.
Acknowledgements
This research was supported in part by the Office of Science (BER), US Department of Energy, grant no. DE-FG02-03ER63602); the National Institute of Health, grant no. SR21CA100092; Congressionally Directed Medical Research Programs (CDMRP; grant no. W81XWH-06-1-0649) and grant-in-aid for Scientific Research (19659315, 19591434, 17659374); and the 21st Century Center of Excellence (COE) Program entitled ‘Establishment of individualized cancer therapy based on comprehensive development of minimally invasive and innovative therapeutic methods (Keio University)’ from the Japanese Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Wang, Y., Nakamura, K. et al. Cell studies of a three-component antisense MORF/tat/Herceptin nanoparticle designed for improved tumor delivery. Cancer Gene Ther 15, 126–132 (2008). https://doi.org/10.1038/sj.cgt.7701111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701111
Keywords
This article is cited by
-
Radioimmunotherapy of human tumours
Nature Reviews Cancer (2015)
-
Tumor delivery of antisense oligomer using trastuzumab within a streptavidin nanoparticle
European Journal of Nuclear Medicine and Molecular Imaging (2009)
-
A Convenient Thiazole Orange Fluorescence Assay for the Evaluation of DNA Duplex Hybridization Stability
Molecular Imaging and Biology (2009)