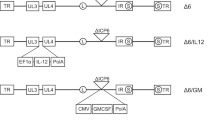
Abstract
Replication competent oncolytic herpes simplex viruses (HSV) with broad-spectrum activity against various cancers, including prostate cancer, exert a dual effect by their direct cytocidal action and by eliciting tumor-specific immunity. These viruses can deliver immunoregulatory molecules to tumors so as to enhance the cumulative antitumor response. This is particularly desirable for prostate cancers, which are usually poorly immunogenic. Initial studies described herein comparing the efficacy of three different oncolytic HSVs (G207, G47Δ, and NV1023) to inhibit the growth of the poorly immunogenic TRAMP-C2 mouse prostate tumors demonstrated that NV1023 was most effective in treating established tumors. The expression of IL-12 on an NV1023 background (NV1042), but not the expression of GM-CSF (NV1034), further enhanced the efficacy of NV1023 in two murine prostate cancer models with highly variable MHC class I levels, Pr14-2 with 91% and TRAMP-C2 with 2% of cells staining. NV1042 also inhibited the growth of distant noninoculated tumors in both prostate cancer models. NV1042 treated tumors exhibited increased immune cell infiltration and decreased levels of angiogenesis. Thus, an IL-12 expressing oncolytic herpes virus, which is capable of direct cytotoxicity and can modulate the otherwise suboptimal immune response through concomitant expression of the cytokine at the site of tumor destruction, could serve as a valuable clinical agent to seek out both overt and occult prostate cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kitano H . Cancer as a robust system: implications for anticancer therapy. Nat Rev Cancer 2004; 4: 227–235.
Rodriguez PC, Zea AH, Ochoa AC . Mechanisms of tumor evasion from the immune response. Cancer Chemother Biol Response Modif 2003; 21: 351–364.
Markiewicz MA, Kast WM . Advances in immunotherapy for prostate cancer. Adv Cancer Res 2003; 87: 159–194.
Strohmeyer DM, Berger AP, Moore II DH, Bartsch G, Klocker H, Carroll PR et al. Genetic aberrations in prostate carcinoma detected by comparative genomic hybridization and microsatellite analysis: association with progression and angiogenesis. Prostate 2004; 59: 43–58.
Leibovitz A, Baumoehl Y, Segal R . Increased incidence of pathological and clinical prostate cancer with age: age related alterations of local immune surveillance. J Urol 2004; 172: 435–437.
Landis SH, Murray T, Bolden S, Wingo PA . Cancer statistics, 1999. CA Cancer J Clin 1999; 49: 8–31.
Assikis VJ, Simons JW . Novel therapeutic strategies for androgen-independent prostate cancer: an update. Semin Oncol 2004; 31: 26–32.
Martuza RL . Conditionally replicating herpes vectors for cancer therapy. J Clin Invest 2000; 105: 841–846.
Varghese S, Rabkin SD . Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther 2002; 9: 967–978.
Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy 2000; 7: 867–874.
Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy 2000; 7: 859–866.
Fong Y, Kemeny N, Jarnagin W, Stanziale S, Guilfoyle B, Gusani N et al. Phase I study of a replication competent herpes simplex oncolytic virus for treatment of hepatic colorectal metastases. Am Soc Clin Oncol 2002; 21: Abstract 8a.
Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL . Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1: 938–943.
Varghese S, Newsome JT, Rabkin SD, McGeagh K, Mahoney D, Nielsen P et al. Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum Gene Ther 2001; 12: 999–1010.
Toda M, Rabkin SD, Kojima H, Martuza RL . Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther 1999; 10: 385–393.
Wong RJ, Patel SG, Kim S, DeMatteo RP, Malhotra S, Bennett JJ et al. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum Gene Ther 2001; 12: 253–265.
Meignier B, Longnecker R, Roizman B . In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis 1988; 158: 602–614.
York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC . A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 1994; 77: 525–535.
Todo T, Martuza RL, Rabkin SD, Johnson PA . Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA 2001; 98: 6396–6401.
Walker JR, McGeagh KG, Sundaresan P, Jorgensen TJ, Rabkin SD, Martuza RL . Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther 1999; 10: 2237–2243.
Cozzi PJ, Burke PB, Bhargav A, Heston WD, Huryk B, Scardino PT et al. Oncolytic viral gene therapy for prostate cancer using two attenuated, replication-competent, genetically engineered herpes simplex viruses. Prostate 2002; 53: 95–100.
Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 1995; 92: 3439–3443.
Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM . Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 1997; 57: 3325–3330.
Maroulakou IG, Anver M, Garrett L, Green JE . Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA 1994; 91: 11236–11240.
Jorcyk CL, Liu ML, Shibata MA, Maroulakou IG, Komschlies KL, McPhaul MJ et al. Development and characterization of a mouse prostate adenocarcinoma cell line: ductal formation determined by extracellular matrix. Prostate 1998; 34: 10–22.
Grossmann ME, Wood M, Celis E . Expression, specificity and immunotherapy potential of prostate-associated genes in murine cell lines. World J Urol 2001; 19: 365–370.
Jarnagin WR, Zager JS, Klimstra D, Delman KA, Malhotra S, Ebright M et al. Neoadjuvant treatment of hepatic malignancy: an oncolytic herpes simplex virus expressing IL-12 effectively treats the parent tumor and protects against recurrence-after resection. Cancer Gene Ther 2003; 10: 215–223.
Fruh K, Yang Y . Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol 1999; 11: 76–81.
Liu R, Varghese S, Rabkin SD . Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res 2005; 65: 1532–1540.
Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR . Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest 2004; 114: 560–568.
Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H et al. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol 2003; 170: 2026–2030.
Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL . Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology 1995; 46: 681–686; discussion 686–687.
Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W et al. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate 1997; 33: 233–239.
Naoe M, Marumoto Y, Ishizaki R, Ogawa Y, Nakagami Y, Yoshida H . Correlation between major histocompatibility complex class I molecules and CD8+ T lymphocytes in prostate, and quantification of CD8 and interferon-gamma mRNA in prostate tissue specimens. BJU Int 2002; 90: 748–753.
Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst 1995; 87: 280–285.
Park JI, Lee MG, Cho K, Park BJ, Chae KS, Byun DS et al. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene 2003; 22: 4314–4332.
Lopez C . Genetics of natural resistance to herpesvirus infections in mice. Nature 1975; 258: 152–153.
Takakuwa H, Goshima F, Nozawa N, Yoshikawa T, Kimata H, Nakao A et al. Oncolytic viral therapy using a spontaneously generated herpes simplex virus type 1 variant for disseminated peritoneal tumor in immunocompetent mice. Arch Virol 2003; 148: 813–825.
Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y . Cytokines in cancer immunity and immunotherapy. Immunol Rev 2004; 202: 275–293.
Bennett JJ, Malhotra S, Wong RJ, Delman K, Zager J, St-Louis M et al. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Ann Surg 2001; 233: 819–826.
Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM . Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA 2000; 97: 2208–2213.
Alatrash G, Hutson TE, Molto L, Richmond A, Nemec C, Mekhail T et al. Clinical and immunologic effects of subcutaneously administered interleukin-12 and interferon alfa-2b: phase I trial of patients with metastatic renal cell carcinoma or malignant melanoma. J Clin Oncol 2004; 22: 2891–2900.
Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res 1997; 3: 409–417.
Cohen J . IL-12 deaths: explanation and a puzzle. Science 1995; 270: 908.
Trinchieri G . Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3: 133–146.
Trinchieri G, Pflanz S, Kastelein RA . The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 2003; 19: 641–644.
Masiero L, Figg WD, Kohn EC . New anti-angiogenesis agents: review of the clinical experience with carboxyamido-triazole (CAI), thalidomide, TNP-470 and interleukin-12. Angiogenesis 1997; 1: 23–35.
Wong RJ, Chan MK, Yu Z, Ghossein RA, Ngai I, Adusumilli PS et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res 2004; 10: 4509–4516.
Jin F, Xie Z, Kuo CJ, Chung LW, Hsieh CL . Cotargeting tumor and tumor endothelium effectively inhibits the growth of human prostate cancer in adenovirus-mediated antiangiogenesis and oncolysis combination therapy. Cancer Gene Ther 2005; 12: 257–267.
Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA 1999; 96: 2233–2238.
Tanaka K, Towata S, Nakao K, Mizuguchi H, Hayakawa T, Niwa M et al. Thyroid cancer immuno-therapy with retroviral and adenoviral vectors expressing granulocyte macrophage colony stimulating factor and interleukin-12 in a rat model. Clin Endocrinol (Oxf) 2003; 59: 734–742.
Gao JQ, Sugita T, Kanagawa N, Iida K, Eto Y, Motomura Y et al. A single intratumoral injection of a fiber-mutant adenoviral vector encoding interleukin 12 induces remarkable anti-tumor and anti-metastatic activity in mice with Meth-A fibrosarcoma. Biochem Biophys Res Commun 2005; 328: 1043–1050.
Triozzi PL, Strong TV, Bucy RP, Allen KO, Carlisle RR, Moore SE et al. Intratumoral administration of a recombinant canarypox virus expressing interleukin 12 in patients with metastatic melanoma. Hum Gene Ther 2005; 16: 91–100.
Iizuka Y, Suzuki A, Kawakami Y, Toda M . Augmentation of antitumor immune responses by multiple intratumoral inoculations of replication-conditional HSV and interleukin-12. J Immunother 2004; 27: 92–98.
Wong RJ, Chan MK, Yu Z, Kim TH, Bhargava A, Stiles BM et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res 2004; 10: 251–259.
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993; 90: 3539–3543.
Tani K, Azuma M, Nakazaki Y, Oyaizu N, Hase H, Ohata J et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther 2004; 10: 799–816.
Nemunaitis J, Sterman D, Jablons D, Smith II JW, Fox B, Maples P et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004; 96: 326–331.
Moret-Tatay I, Diaz J, Marco FM, Crespo A, Alino SF . Complete tumor prevention by engineered tumor cell vaccines employing nonviral vectors. Cancer Gene Ther 2003; 10: 887–897.
Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol 2003; 21: 3343–3350.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I . High-dose granulocyte–macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 2004; 64: 6337–6343.
Acknowledgements
We thank Tomoki Todo for his valuable scientific assistance, and Wenzheng Zhang, Dana Xu, Thanh Thao Huynh and Usha Macgarvey for their technical assistance. RLM and SDR are consultants to MediGene AG, which has a license from Georgetown University to commercialize G207. This research was supported by grants to RLM from NIH (1R01CA102139-01A1), Department of Defense (DAMD17-98-1-8490), and the CapCure Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varghese, S., Rabkin, S., Liu, R. et al. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther 13, 253–265 (2006). https://doi.org/10.1038/sj.cgt.7700900
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700900
Keywords
This article is cited by
-
Fusion peptide is superior to co-expressing subunits for arming oncolytic herpes virus with interleukin 12
Communications Medicine (2023)
-
Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1
Gene Therapy (2021)
-
Oncolytic herpes simplex virus and immunotherapy
BMC Immunology (2018)
-
Potentiating prostate cancer immunotherapy with oncolytic viruses
Nature Reviews Urology (2018)
-
Driving cars to the clinic for solid tumors
Gene Therapy (2018)