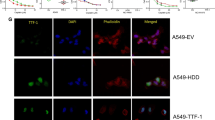
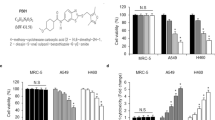
Abstract
While external ionizing radiation has been used for treating non-small cell lung cancer (NSCLC), improved efficacy of this modality would be an important advance. Ectopic expression of the sodium iodide symporter (NIS) and thyroperoxidase (TPO) genes in NSCLC cells facilitated concentration of iodide in NSCLC cells, which markedly induced apoptosis in vitro and in vivo. Pre-incubation of the NIS/TPO-modified NSCLC cells in iodide followed by ionizing radiation generates bystander tumoricidal effects and potently enhances tumor cell killing. This iodide-induced bystander effect is associated with enhanced gap junction intercellular communication (GJIC) activity and increased connexin-43 (Cx43) expression. Thus, iodide may serve as an enhancer to markedly improve the efficacy of radiation therapy in combined therapeutic modalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Corn BW . Advances in management of malignant diseases with the combination of radiation therapy and chemotherapy. Drugs Today (Barc) 2004; 40: 71–79.
Komaki R, Liao Z, Milas L . Improvement strategies for molecular targeting: cyclooxygenase-2 inhibitors as radiosensitizers for non-small cell lung cancer. Semin Oncol 2004; 31: 47–53.
Zhang M, Boyer M, Rivory L, Hong A, Clarke S, Stevens G et al. Radiosensitization of vinorelbine and gemcitabine in NCI-H460 non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys 2004; 58: 353–360.
Dai G, Levy O, Carrasco N . Cloning and characterization of the thyroid iodide transporter. Nature 1996; 379: 458–460.
Kaminsky SM, Levy O, Salvador C, Dai G, Carrasco N . Na(+)-I- symport activity is present in membrane vesicles from thyrotropin-deprived non-I(−)-transporting cultured thyroid cells. Proc Natl Acad Sci USA 1994; 91: 3789–3793.
Trapasso F, Iuliano R, Chiefari E, Arturi F, Stella A, Filetti S et al. Iodide symporter gene expression in normal and transformed rat thyroid cells. Eur J Endocrinol 1999; 140: 447–451.
Nilsson M . Molecular and cellular mechanisms of transepithelial iodide transport in the thyroid. Biofactors 1999; 10: 277–285.
Spitzweg C, Morris JC . The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf) 2002; 57: 559–574.
Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M . Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol 1999; 141: 443–457.
Huang M, Batra RK, Kogai T, Lin YQ, Hershman JM, Lichtenstein A et al. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non-small cell lung cancer. Cancer Gene Ther 2001; 8: 612–618.
Zhang L, Sharma S, Zhu LX, Kogai T, Hershman JM, Brent GA et al. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res 2003; 63: 5065–5072.
Mandell RB, Mandell LZ, Link Jr CJ . Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res 1999; 59: 661–668.
Gaut AW, Niu G, Krager KJ, Graham MM, Trask DK, Domann FE . Genetically targeted radiotherapy of head and neck squamous cell carcinoma using the sodium-iodide symporter (NIS). Head Neck 2004; 26: 265–271.
Mitrofanova E, Hagan C, Qi J, Seregina T, Link Jr C . Sodium iodide symporter/radioactive iodine system has more efficient antitumor effect in three-dimensional spheroids. Anticancer Res 2003; 23: 2397–2404.
Haberkorn U, Kinscherf R, Kissel M, Kubler W, Mahmut M, Sieger S et al. Enhanced iodide transport after transfer of the human sodium iodide symporter gene is associated with lack of retention and low absorbed dose. Gene Ther 2003; 10: 774–780.
Imaizumi K, Hasegawa Y, Kawabe T, Emi N, Saito H, Naruse K et al. Bystander tumoricidal effect and gap junctional communication in lung cancer cell lines. Am J Respir Cell Mol Biol 1998; 18: 205–212.
Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB . Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 2001; 61: 3894–3901.
Slupphaug G, Kavli B, Krokan HE . The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 2003; 531: 231–251.
Carlin S, Cunningham SH, Boyd M, McCluskey AG, Mairs RJ . Experimental targeted radioiodide therapy following transfection of the sodium iodide symporter gene: effect on clonogenicity in both two-and three-dimensional models. Cancer Gene Ther 2000; 7: 1529–1536.
Shao C, Furusawa Y, Aoki M, Ando K . Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat Res 2003; 160: 318–323.
Ruch RJ, Porter S, Koffler LD, Dwyer-Nield LD, Malkinson AM . Defective gap junctional intercellular communication in lung cancer: loss of an important mediator of tissue homeostasis and phenotypic regulation. Exp Lung Res 2001; 27: 231–243.
Franceschi S, Bidoli E . The epidemiology of lung cancer. Ann Oncol 1999; 10(Suppl 5): S3–S6.
Pandey M, Mathew A, Nair MK . Global perspective of tobacco habits and lung cancer: a lesson for third world countries. Eur J Cancer Prev 1999; 8: 271–279.
Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker Jr S et al. American Society of Clinical Oncology Treatment of Unresectable Non-Small-Cell Lung Cancer Guideline: Update 2003. J Clin Oncol 2003; 22: 330–353.
Saijo N . Irinotecan Combined with Radiation Therapy for Patients with Stage III Non-Small-Cell Lung Cancer: Current Trials. Clin Lung Cancer 2002; 4(Suppl 1): S21–S25.
Kada T, Noguti T, Namiki M . Radio-sensitization with iodine compounds. I. Examination of damage in deoxyribonucleic acid with Bacillus subtilis transformation system by irradiation in the presence of potassium iodide. Int J Radiat Biol Relat Stud Phys Chem Med 1970; 17: 407–418.
Kada T, Sadaie Y, Noguti T . Radio-sensitization with extra-cellular halogenated purines and pyrimidines and related compounds. Int J Radiat Biol Relat Stud Phys Chem Med 1970; 18: 281–285.
Mikkelsen RB, Wardman P . Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003; 22: 5734–5754.
Matono S, Tanaka T, Sueyoshi S, Yamana H, Fujita H, Shirouzu K . Bystander effect in suicide gene therapy is directly proportional to the degree of gap junctional intercellular communication in esophageal cancer. Int J Oncol 2003; 23: 1309–1315.
Culver KW, Vickers TM, Lamsam JL, Walling HW, Seregina T . Gene therapy for solid tumors. Br Med Bull 1995; 51: 192–204.
Pitts JD . Cancer gene therapy: a bystander effect using the gap junctional pathway. Mol Carcinog 1994; 11: 127–130.
Kolaja KL, Petrick JS, Klaassen CD . Inhibition of gap-junctional-intercellular communication in thyroid-follicular cells by propylthiouracil and low iodine diet. Toxicology 2000; 143: 195–202.
Ruch RJ, Cesen-Cummings K, Malkinson AM . Role of gap junctions in lung neoplasia. Exp Lung Res 1998; 24: 523–539.
Cesen-Cummings K, Fernstrom MJ, Malkinson AM, Ruch RJ . Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis 1998; 19: 61–67.
Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H . Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA 1996; 93: 1831–1835.
Krutovskikh VA, Piccoli C, Yamasaki H . Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 2002; 21: 1989–1999.
Mothersill C, Seymour C . Survival of human epithelial cells irradiated with cobalt 60 as microcolonies or single cells. Int J Radiat Biol 1997; 72: 597–606.
Acknowledgements
This work is supported by The UCLA SPORE in Lung Cancer, National Institutes of Health P50 CA90388, R01 CA085686 (SMD), Medical Research Funds from the Department of Veteran Affairs, and the Tobacco-Related Disease Research Program of the University of California.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Sharma, S., Hershman, J. et al. Iodide sensitizes genetically modified non-small cell lung cancer cells to ionizing radiation. Cancer Gene Ther 13, 74–81 (2006). https://doi.org/10.1038/sj.cgt.7700875
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700875
Keywords
This article is cited by
-
Transfection of the human sodium/iodide symporter (NIS) gene with liposomes and the expression of the NIS protein in human lung A549 cancer cells
Chinese Journal of Clinical Oncology (2008)