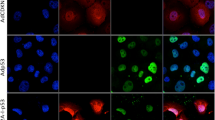
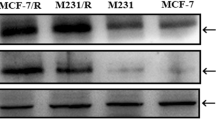
Abstract
Tumor suppressor p53-based gene therapy strategy is ineffective in certain conditions. p73, a p53 homologue, could be a potential alternative gene therapy agent as it has been found to be an important determinant of chemosensitivity in cancer cells. Previously, we have reported the generation of a replication-deficient adenovirus expressing p73β (Ad-p73). In this study, we evaluated the therapeutic potential of Ad-p73 against a panel of cancer cells (n=12) of different tissue origin. Ad-p73 infected all the cell lines tested very efficiently resulting in several-fold increase in p73β levels, which is also functional as it activated the known target gene p21WAF1/CIP1. Infection with Ad-p73 resulted in potent cytotoxicity in all the cell lines tested. The mechanism of p73-induced cytotoxicity in these cell lines is found to be due to a combination of cell cycle arrest and induction of apoptosis. In addition, exogenous overexpression of p73 by Ad-p73 infection increased the chemosensitivity of cancer cells by many fold to commonly used drug adriamycin. Moreover, Ad-p73 is more efficient than Ad-p53 in enhancing the chemosensitivity of mutant p53 harboring cells. Furthermore, Ad-p73 infection did not induce apoptosis in human normal lung fibroblasts (HEL 299) and human immortalized keratinocytes (HaCaT). These results suggest that Ad-p73 is a potent cytotoxic agent specifically against cancer cells and could be developed as a cancer gene therapy agent either alone or in combination with chemotherapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- wt:
-
wild-type
- mut:
-
mutant
- BrdU:
-
bromodeoxyuridine
- MOI:
-
multiplicity of infection
References
Oliner JD, Pientenpol JA, Thiagalingam S, et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860.
Haupt YR, Maya R, Kaza A, Oren M . MDM2 promotes the rapid degradation of p53. Nature. 1997;387:296–299.
Kubbutat MH, Jones SN, Vousden K . Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303.
Kamijo T, Weber JD, Zambetti G, et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA. 1998;5:8292–8297.
Stott FJ, Bates S, James MC, et al. The alternative product from the human CDKN2A locus, p14 (ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014.
Zhang Y, Xiong Y, Yarbrough WG . ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734.
Orlow I, LaRue H, Osman I, et al. Deletions of the INK4A gene in superficial bladder tumors. Association with recurrence. Am J Pathol. 1999;155:105–113.
Roth JA, Nguyen D, Lawrence DD, et al. Retrovirus mediated wildtype p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996;2:985–991.
Swisher SG, Roth JA, Nemunaitis J, et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:763–771.
Nemunaitis J, Swisher SG, Timmons T, et al. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small cell lung cancer. J Clin Oncol.. 2000;18:609–622.
Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819.
Jost CA, Marin MC, Kaelin WG . p73 is a human p53-related protein that can induce apoptosis. Nature. 1997;389:191–194.
Agami R, Blandino G, Oren M, et al. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature. 1999;399:809–813.
Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809.
Yuan ZM, Shioya H, Ishiko T, et al. p73 is regulated by tyrosine kinase c-abl in apoptotic response to DNA damage. Nature. 1999;399:814–817.
Chen X, Zheng Y, Zhu J, et al. p73 is transcriptionally regulated by DNA damage, p53 and p73. Oncogene. 2001;20:769–774.
Costanzo A, Merlo P, Pediconi N, et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol Cell. 2002;9:175–186.
Irwin MS, Kondo K, Marin MC, et al. Chemosensitivity linked to p73 function. Cancer Cell. 2003;2:403–410.
Flores ER, Tsai KY, Crowley D, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564.
Das S, El-Deiry WS, Somasundaram K . Efficient growth inhibition of HPV E6-expressing cells by an adenovirus expressing p53 homologue p73β. Oncogene. 2003;22:8394–8402.
Sasaki Y, Morimoto S, Ishida Y, et al. Adenovirus mediated transfer of the p53 family genes, p73 and p51/p63 induces cell cycle arrest and apoptosis in colorectal cancer cell lines: potential implications to gene therapy of colorectal cancer. Gene Therapy. 2001;8:1401–1408.
Somasundaram K, Zhang H, Zeng YX, et al. Arrest of the cell cycle by thee tumor suppressor BRCA1 requires CDK-inhibitor p21WAF1/CIP1. Nature. 1997;389:187–190.
Somasundaram K, MacLachlan TK, Burns TF, et al. BRCA1 signals ARF-dependent stabilization and coactivation of p53. Oncogene. 2000;18:6605–6614.
Das S, El-Deiry WS, Somasundaram K . Regulation of p53 homolog p73 by adenoviral oncogene E1A. J Biol Chem. 2003;278:18313–18320.
El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75: 817–825.
Somasundaram K, El-Deiry WS . Inhibition of p53- mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene. 1997;14:1047–1105.
Blagosklonny M, El-Deiry WS . In vitro evaluation of a p53-expressing adenovirus as an anti-cancer drug. Int J Cancer. 1996;67:386–392.
Wajapeyee N, Somasundaram K . Cell cycle arrest and apoptosis induction by Activator Protein 2 α (AP-2 α) and the role of p53 and p21WAF1/CIP1 in AP-2 α-mediated growth inhibition. J Biol Chem.. 2003;52:52093–52101.
Prabhu NS, Somasundaram K, Satyamoorthy K, et al. p73β, unlike p53, suppresses growth and induces apoptosis of human papillomvirus E6-expressing cancer cells. Int J Oncol. 1998;13:5–9.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . p53 mutations in human cancers. Science. 1991;253:49–53.
Levine AJ . p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331.
Storey A, Thomas M, Kalita A, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234.
Lechner MA, Laimins LA . Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J. Virol. 1994;68: 4262–4273.
Beer-Romero P, Glass S, Rolfe M . Antisense targeting of E6AP elevates p53 in HPV-infected cells but not in normal cells. Oncogene. 1997;14:595–602.
Di Commo, Gaiddon C, Prives C . p73 function is inhibited by tumor derived p53 mutants in mammalian cells. Mol Cell Biol. 1999;19:1438–1449.
Gaiddon C, Lokshin M, Ahn J, et al. A subset of tumor-derived mutant forms of p53 downregulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–1887.
Acknowledgements
We thank Dr Omana Joy for technical assistance in FACS experiments. This study was supported partly by grants from Life Science Research Board, DRDO, and Council of Scientific and Industrial Research (CSIR), Government of India. Funding from ICMR (Center for Advanced studies in Molecular Medicine), DBT (Program support), DST (FIST) and UGC (Special assistance) to Department of Microbiology and Cell Biology is also acknowledged. KS is a Wellcome Trust International Senior Research Fellow. SD, SN, and SA are supported by fellowships from CSIR, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, S., Nama, S., Antony, S. et al. p73β-expressing recombinant adenovirus: a potential anticancer agent. Cancer Gene Ther 12, 417–426 (2005). https://doi.org/10.1038/sj.cgt.7700803
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700803
Keywords
This article is cited by
-
Gene therapy in India: A focus
Journal of Biosciences (2014)
-
Functions, divergence and clinical value of TAp73 isoforms in cancer
Cancer and Metastasis Reviews (2013)
-
p73 is essential for vitamin D-mediated osteoblastic differentiation
Cell Death & Differentiation (2010)
-
The role of p73 in hematological malignancies
Leukemia (2006)