Abstract
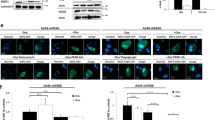
One important feature of human solid tumors is the presence of a hypoxic microenvironment. Under hypoxia, genes that contain a hypoxia-response element (HRE) can be activated by the binding of hypoxia-inducible factor-1. To reach the goal of selectively killing tumor cells in a hypoxic microenvironment using a gene therapy approach, we developed a cytosine deaminase (CD) gene construct (pH9YCD2) that contains an HRE gene enhancer. CD is an enzyme that catalyzes the conversion of noncytotoxic 5-fluorocytosine (5-FC) to the cytotoxic and radiosensitizing drug 5-fluorouracil (5-FU). Yeast CD was cloned into an SV40 promoter-based mammalian expression vector, and an HRE enhancer was inserted in front of the promoter. Human glioblastoma U-87 MG cells were transfected with pH9YCD2. Western blots revealed that CD was strongly expressed under hypoxic conditions (0.3–1% O2), whereas only minor CD expression was seen under normoxic conditions. To confirm that the expressed CD enzyme retains catalytic activity, we performed a 5-FC/5-FU-conversion assay in which 5-FC was incubated with the lysates of pH9YCD2-transfected cells. The percentage of conversion from 5-FC to 5-FU was 63% under hypoxia versus 13% under normoxia. In vitro, cell viability and colony-forming efficiency assays demonstrated that the gene construct was able to significantly kill glioblastoma cells in a hypoxia-dependent manner. In addition, 5-FC treatment of hypoxic pH9YCD2-transfected cells produced a marked bystander effect, which could be a distinct advantage for gene therapy. If this construct exhibits antitumor efficacy in vivo, it may have promise as an antitumor agent in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vaupel P, Kallinowski F, Okunieff P . Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465.
Hockel M, Vaupel P . Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276.
Rampling R, Cruickshank G, Lewis AD, et al. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29:427–431.
Kayama T, Yoshimoto T, Fujimoto S, Sakurai Y . Intratumoral oxygen pressure in malignant brain tumor. J Neurosurg. 1991;74:55–59.
Brahimi-Horn C, Berra E, Pouyssegur J . Hypoxia: the tumor's gateway to progression along the angiogenic pathway. Trends Cell Biol. 2001;11:S32–S36.
Brown JM . The hypoxic cell: a target for selective cancer therapy — eighteenth Bruce F Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870.
Ruan H, Su H, Hu L, et al. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3:255–263.
Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515.
Zagzag D, Zhong H, Scalzitti JM, et al. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618.
Semenza GL . HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67.
Ferrara N, Houck K, Jakeman L, Leung DW . Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32.
Seagroves TN, Ryan HE, Lu H, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21:3436–3444.
Semenza GL . Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350.
Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468.
Min JH, Yang H, Ivan M, et al. Structure of an HIF-1alpha–pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889.
Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472.
Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421.
Zhong H, DeMarzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835.
Minchenko A, Leshchinsky I, Opentanova I, et al. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem. 2002;277:6183–6187.
Ruan H, Wang J, Hu L, et al. Killing of brain tumor cells by hypoxia-responsive element mediated expression of BAX. Neoplasia. 1999;1:431–437.
Hassouna I, Wickert H, Zimmermann M, Gillardon F . Increase in bax expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci Lett. 1996;204:85–88.
Pastorino JG, Chen ST, Tafani M, et al. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775.
Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T . Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640.
Corban-Wilhelm H, Hull WE, Becker G, et al. Cytosine deaminase and thymidine kinase gene therapy in a Dunning rat prostate tumour model: absence of bystander effects and characterisation of 5-fluorocytosine metabolism with 19F-NMR spectroscopy. Gene Ther. 2002;9:1564–1575.
Heidelberger C, Danenberg PV, Moran RG . Fluorinated pyrimidines and their nucleosides. Adv Enzymol Relat Areas Mol Biol. 1983;54:58–119.
Hamstra DA, Rice DJ, Fahmy S, et al. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase. Hum Gene Ther. 1999;10:1993–2003.
Kievit E, Bershad E, Ng E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421.
Khil MS, Kim JH, Mullen CA, et al. Radiosensitization by 5-fluorocytosine of human colorectal carcinoma cells in culture transduced with cytosine deaminase gene. Clin Cancer Res. 1996;2:53–57.
Vauthey JN, Marsh Rde W, Cendan JC, et al. Arterial therapy of hepatic colorectal metastases. Br J Surg. 1996;83:447–455.
Dachs GU, Patterson AV, Firth JD, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520.
Wouters BG, Brown JM . Cells at intermediate oxygen levels can be more important than the ‘hypoxic fraction’ in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–550.
Koshikawa N, Takenaga K, Tagawa M, Sakiyama S . Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res. 2000;60:2936–2941.
Shibata T, Giaccia AJ, Brown JM . Hypoxia-inducible regulation of a prodrug-activating enzyme for tumor-specific gene therapy. Neoplasia. 2002;4:40–48.
Cao YJ, Shibata T, Rainov NG . Hypoxia-inducible transgene expression in differentiated human NT2N neurons — a cell culture model for gene therapy of postischemic neuronal loss. Gene Ther. 2001;8:1357–1362.
Gupta N, Vij R, Haas-Kogan DA, et al. Cytogenetic damage and the radiation-induced G1-phase checkpoint. Radiat Res. 1996;145:289–298.
Lawrence TS, Rehemtulla A, Ng EY, et al. Preferential cytotoxicity of cells transduced with cytosine deaminase compared to bystander cells after treatment with 5-flucytosine. Cancer Res. 1998;58:2588–2593.
Erbs P, Exinger F, Jund R . Characterization of the Saccharomyces cerevisiae FCY1 gene encoding cytosine deaminase and its homologue FCA1 of Candida albicans. Curr Genet. 1997;31:1–6.
Kozak M . An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148.
Semenza GL, Roth PH, Fang HM, Wang GL . Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763.
Erbs P, Regulier E, Kintz J, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822.
Acknowledgements
We thank Dr Philippe Erbs, Transgene SA, Strasbourg Cedex, France, for providing technical assistance for the cytosine deaminase activity assay. We are also grateful to Sharon Reynolds, Department of Neurological Surgery, University of California at San Francisco, for editorial assistance. This work was supported by NIH Grants CA-85356 (to DFD), NS-42927 (to DFD) and CA-85878 (to AR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Ruan, H., Hu, L. et al. Development of a hypoxia-inducible cytosine deaminase expression vector for gene-directed prodrug cancer therapy. Cancer Gene Ther 12, 276–283 (2005). https://doi.org/10.1038/sj.cgt.7700748
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700748
Keywords
This article is cited by
-
Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target
Journal of Neuro-Oncology (2009)