Abstract
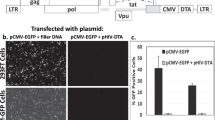
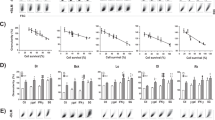
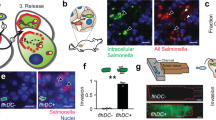
The bacterial enzyme carboxypeptidase G2 (CPG2) can be expressed both intracellularly (CPG2*) or tethered to the outer surface (stCPG2(Q)3) of mammalian cells, where it is able to activate mustard prodrugs for use in suicide gene therapy protocols. Here we compare the properties of CPG2 expressed in these two locations. CPG2 is active as a dimer, and one of the mutations required to block glycosylation of stCPG2(Q)3 destabilizes the dimers. Some of the mutations to this site partially correct the dimerization defect and recover a proportion of the activity. Surface tethering also recovers some enzyme activity, but through an unknown mechanism. The efficacy of CPG2 in these two locations is compared with the tumor cell lines A2780, SK-OV-3, and WiDr, which are sensitized to the prodrug 4-([2-chloroethyl][2-mesyloxyethyl]amino)benzoyl-L-glutamic acid (CMDA) by both CPG2* and stCPG2(Q)3 expression in suicide gene therapy protocols in vitro. We find that stCPG2(Q)3 is a more efficient mediator of CMDA-dependent cell killing than CPG2*. Lower levels of stCPG2(Q)3 activity are required to give cell killing that can only be achieved by higher levels of CPG2*. In bystander effect assays, low levels of stCPG2(Q)3 are required for efficient killing, whereas relatively high levels of CPG2* activity are required. Also, shorter exposures to prodrug are required for cell killing when stCPG2(Q)3 is expressed compared with when CPG2* is expressed. These data demonstrate that the location of the enzyme in the cell is more important than the enzyme activity as the determinant in mediating cytotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spooner, R., Martin, J., Friedlos, F. et al. In suicide gene therapy, the site of subcellular localization of the activating enzyme is more important than the rate at which it activates prodrug. Cancer Gene Ther 7, 1348–1356 (2000). https://doi.org/10.1038/sj.cgt.7700243
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700243
Keywords
This article is cited by
-
Adenoviral vector-mediated expression of a gene encoding secreted, EpCAM-targeted carboxylesterase-2 sensitises colon cancer spheroids to CPT-11
British Journal of Cancer (2005)
-
Hapten-directed targeting to single-chain antibody receptors
Cancer Gene Therapy (2004)
-
A novel vascular endothelial growth factor-directed therapy that selectively activates cytotoxic prodrugs
British Journal of Cancer (2003)
-
Adenovirus vector–mediated delivery of the prodrug-converting enzyme carboxypeptidase G2 in a secreted or GPI-anchored form: High-level expression of this active conditional cytotoxic enzyme at the plasma membrane
Cancer Gene Therapy (2002)
-
Expression of the prodrug-activating enzyme DT-diaphorase via Ad5 delivery to human colon carcinoma cells in vitro
Cancer Gene Therapy (2002)