Abstract
Urokinase plasminogen activator receptor (uPAR) plays a major role in cancer invasion and metastasis and uPAR expression is correlated with a poor prognosis in various cancer types. Moreover, the expression of uPAR is increased under hypoxic conditions. Nitric oxide (NO) and its metabolites produced by inducible nitric oxide synthase (iNOS) are important products of hypoxic stress, and NO may activate or modulate extracellular signal regulated kinase (ERK). Here, we evaluated uPA, uPAR, and activated ERK levels under hypoxic conditions, and the modulatory effects of iNOS and NO in the MDA-MB-231 human breast cancer cell line. Cells were incubated in a hypoxic or normoxic incubator and treated with PD98059 (a MEK 1/2 inhibitor, which abrogates ERK phosphorylation) and aminoguanidine (a selective iNOS inhibitor). uPAR expression, ERK phosphorylation, and uPA activity were found to be increased under hypoxic conditions. Moreover, when cells were treated with PD98059 under hypoxic conditions, uPAR was downregulated, whereas aminoguanidine markedly increased ERK phosphorylation in a dose dependent manner. Furthermore, aminoguanidine increased uPAR expression and prevented the inhibition of uPAR expression by PD98059. These results demonstrated that uPAR is induced by hypoxia and that increased uPAR expression is mediated by ERK phosphorylation, which in turn is modulated by iNOS/NO in MDA-MB-231 cells. We conclude that iNOS/NO downregulates the expression of uPAR under hypoxic conditions via ERK pathway modulation.
Similar content being viewed by others
Introduction
Hypoxic stress underlies a number of important biological processes, such as, cellular migration and invasion, and tumor growth 1, 2, 3, 4. For example, hypoxia within an expanding tumor leads to the release of vascular endothelial growth factor (VEGF) and the stimulation of angiogenesis, the success of which depends on endothelial cell migration and invasion 5, 6. Hypoxia may promote cancer metastasis, which has been demonstrated in experimental tumor models 7, 8, 9. However, the detailed mechanism by which hypoxia provokes cancer invasion and metastasis has not been fully elucidated.
Some tumor cell lines have been shown to up-regulate plasminogen activator inhibitor I (PAI-I) and uPAR when cells are exposed to hypoxia in vitro2, 3, 4, 7, 10, 11. The uPAR gene promoter possesses HIF-1α binding sites, and has been shown to be activated by HIF-1α under hypoxic stress in human trophoblasts and in human umbilical vein endothelial cells 2. In the normoxic state, uPA signaling through uPAR was observed to maintain an elevated basal level of activated ERK and to inhibit apoptosis in breast cancer cell lines 12. Moreover, hypoxia induced the up-regulation of uPAR in a human prostate cancer cell line via ERK and p38 kinase signaling pathways 11. The plasminogen activation system, leads to the formation of serine proteinase plasmin, and has been shown to play an important role in metastasis 7, 13, 14. This activation system contains tissue plasminogen activator (tPA), uPA, PAI-1, PAI-2 and uPAR, and uPA is known to localize at the surface of tumor cells by binding to a specific receptor uPAR 15. The uPAR/uPA complex is focused on the formation of plasmin and hence influences proteolytic activity in the vicinity of tumor cells. Plasmin facilitates tumor cell migration, invasion, and metastasis by degrading fibrin and other matrix proteins directly, and by activating several metalloproteinases that also degrade the extracellular matrix 16.
Nitric oxide (NO) and its metabolites, which are produced by nitric oxide synthases (NOSs), are important products of hypoxic stress 17, 18, 19. And, human breast carcinoma cells and mouse mammary tumor cell lines produce NO in amounts correlated with tumor grade and metastasis 20, 21, 22. Moreover, breast cancer patients with an iNOS positive tumor have a poorer outcome than those with an iNOS negative tumor 23. The mechanisms by which NO may enhance mammary tumor development and metastases include increasing DNA damage and tumor cells migration, and promoting angiogenesis 21, 24. NO may activate or modulate MAPK/ERK, G-proteins, the Ras pathway and PI3K signaling 21, 24, 25. However, the molecular interactions between iNOS/NO, ERK, and uPAR in hypoxic states have not been elucidated. In this study, we examined uPAR expression and ERK activation in human MDA-MB-231 breast cancer cells under hypoxic conditions and investigated the roles of ERK, iNOS/NO as upstream regulators of uPAR.
Material and methods
Cell lines and hypoxic culture conditions
The human metastatic breast carcinoma cell line, MDA-MB-231 was used throughout this study. Cells were purchased from KCLB (the Korean Cell Line Bank, Seoul) and cultured in RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. All cells were used at 80% confluence. For cell culture under 1% O2 tension the cells were incubated for 2 h to 48 h in a humidified atmosphere at 37 °C in a multi-gas CO2-O2 incubator (NUAIRE, Plymouth, MN) equilibrated with 1% O2, 5% CO2, and 94% N2. Control cultures were performed in 20% O2 and 5% CO2, in a CO2 incubator (NUAIRE, Plymouth, MN) at 37 °C. Cell harvesting was performed in a hypoxic chamber.
Gel zymography and uPA activity
uPA enzyme activity in culture media was measured by gel zymography. 1×106 cells were seeded in 10 cm2 dishes and when sub-confluent, media were changed to serum free media to exclude the influence of serum factors. After a 3h equilibration period, the cells were incubated in the hypoxic incubator. Media were collected and concentrated with a serum concentrating kit after 4h, 8h, and 12h of hypoxia treatment. A sample volume containing 30μg of protein was mixed with SDS sample buffer and loaded into an SDS/polyacrylamide gel containing 2mg/ml α-casein and 0.025 units/ml of plasminogen (Sigma, St. Louis, MO). Following electrophoresis, gels were washed twice for 10min with 2.5% Triton X-100 (Sigma, St. Louis, MO) in water, rinsed briefly with water, and incubated overnight in a solution of 50mM TRIS and 5mM CaCl2. The gels were then stained with 0.4% Coomassie brilliant blue R-250 in 10% acetic acid/40% methanol, destained in 10% acetic acid/40% methanol, and dried between sheets of cellophane on a gel dryer (Vision, Bucheon, Korea).
Western blot analysis for uPAR, ERK, HIF-1α expression
Cells were washed in PBS, detached using Trypsin-EDTA buffer, and stored at −70 °C. Protein was extracted with RIPA buffer (1% NP-40/Ig PAL, 0.5% sodium deoxycholate, 0.1% SDS) and protease inhibitors (aprotinin, leupeptin, PMSF, benzamidine, trypsin inhibitor, sodium orthovanadate). Total protein was analyzed quantitatively using a spectrophotometer at 595 nm. 30 μg of proteins were then separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Membranes were incubated with anti-uPAR goat polyclonal antibody, and phosphorylated ERK (p-ERK) polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and bound antibody was detected by biotin-streptavidin alkaline phosphatase staining followed by ECL (Amersham, Piscataway, NJ). After 3 min to 30 min, films were developed and analyzed. Blots were probed with anti-β-actin mouse monoclonal antibody (Sigma Chemical Co., St. Louis, MO) as a loading control. Protein molecular weights were estimated using prestained standards, according the manufacturer's instructions.
Drug treatments
To evaluate the contributions of the ERK pathway and NO signaling, we used an MEK 1/2 inhibitor (PD 98059, Sigma, St. Louis, MO), which abrogates ERK phosphorylation, and a selective iNOS inhibitor (aminoguanidine). All drugs were purchased from Sigma (St. Louis, MO)
Results
Hypoxia induced uPAR expression in MDA-MB-231 cells via ERK signaling
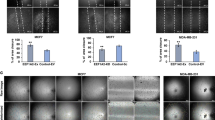
To determine the time course of uPAR expression and ERK activation in MDA-MB-231 cells under hypoxic conditions, cells were cultured in the 1% O2 atmosphere for various times. As expected, the induction of uPAR protein was detected as early as 4 h after exposure to hypoxia and remained elevated for 24 h (Figure 1). In two other time course experiments, we found that the maximal level of uPAR induction was sustained for 48 h (data not shown). To determine whether hypoxia induces ERK phosphorylation in MDA MB 231 cells, we examined ERK phosphorylation at various times following exposure to 1% hypoxia. As was observed for uPAR, phosphorylated ERK levels were elevated under hypoxic conditions (Figure 1B). The induction of uPA activity began at 4 h after exposure to 1% hypoxia and remained until 12 h in MDA-MB-231 cells by gel zymography, though the degree of induction was modest (Figure 1C). Taken together, both p-ERK and uPA/uPAR signals were induced following the exposure of MDA-MB-231 human breast cancer cells to 1% hypoxia. In human epidermoid carcinoma cell lines and human colon carcinoma cell lines, agents that regulate ERK activation were also found to regulate the expressions of uPA and uPAR 26, 27. The ERK pathway is activated after exposure to hypoxia 28, 29, and thus, ERK activation may affect cell invasion and metastasis indirectly by affecting the expressions of uPA and uPAR. To determine whether uPAR expression is dependent on ERK phosphorylation in hypoxic conditions, MDA-MB-231 cells were treated with the MEK1/2 inhibitor PD98059 under 1% hypoxia, and it was found that uPAR expression was markedly decreased (Figure 2B). These data provide evidence that uPAR expression in MDA-MB-231 cells under hypoxic conditions depends on the activation of ERK.
Induction of the uPA/uPAR signal and the phosphorylation of ERK in human MDA-MB-231 cells under hypoxia. (A) uPAR was markedly induced in a 1% O2 environment after 4 to 24 h. (B) ERK phosphorylation was induced after exposure to hypoxia for the indicated times. (C) uPA activity by gel zymography was increased after 4, 8 and 12 h of culture in a hypoxic environment. The data shown represent three separate experiments. N: normoxia.
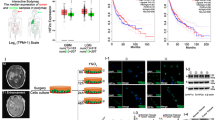
Modulation of uPAR expression by ERK and iNOS/NO in MDA-MB-231 human breast cancer cells under hypoxia. (A) Aminoguanidine, a selective iNOS inhibitor, increased ERK phosphorylation in a dose dependent manner at concentrations of 0.5, 5, and 50 μM in MDA-MB-231 cells exposed to hypoxia for 4 h. ERK phosphorylation was dramatically increased by the selective iNOS inhibitor, aminoguanidine. (B) The MEK 1/2 inhibitor, PD98059 (20 μM) prevented uPAR induction in human MDA-MB-231 breast cancer cells exposed to hypoxia for 4h (lane 2). 5 μM of aminoguanidine prevented the inhibition of uPAR by PD98059 and increased uPAR expression in MDA-MB231 cells under hypoxic stress (3rd and 4th lanes). The data shown represent three separate experiments. AG: aminoguanidine. Blotting with anti b-actin antibody showed that lanes were equally loaded.
The selective iNOS inhibitor, aminoguanidine, increased uPAR expression by modulating the ERK pathway
To test whether iNOS/NO modulates ERK phosphorylation in MDA-MB-231 cells under hypoxic conditions, cultures were treated with aminoguanidine under 1% hypoxic conditions for 4 h. As shown Figure 2A, aminoguanidine induced ERK phosphorylation in a dose dependent manner in MDA-MB-231 cells. We hypothesized that inhibiting iNOS would upregulate hypoxia-induced uPAR expression via the ERK pathway. As was expected, aminoguanidine prevented the inhibition of uPAR by PD98059 (3rd lane) and increased uPAR expression (4th lane). Taken together these results suggest that NO decreases uPAR expression by inhibiting ERK dependent and independent pathways.
Discussion
The major finding of the present study is that uPAR induction in MDA-MB-231 human breast cancer cells under hypoxia depends on the ERK signaling pathway, and which is regulated by the iNOS/NO pathway. Moreover, iNOS and NO had an effect on uPAR induction under hypoxia through both an ERK dependent and an ERK independent pathway (Figure 3 ).
Schematic diagram of the uPA-uPAR regulatory system. It is well known that hypoxia increases the invasion and migration of malignant tumor cells by activating uPA/uPAR signaling via HIF-1α transcription 2, 3, 4, 7, 34. NO and its metabolites produced by iNOS are important products of hypoxic stress 17, 18, 19, 20, and ERK activation is known to be needed for HIF-1α stabilization 28, 29, 45 and HIF-1α activation 29. In the present study shows that uPA/uPAR signaling was modulated by the ERK and iNOS/NO pathways in human MDA-MB-231 cells under hypoxic stress. Hypoxia induced uPAR expression in human MDA-MB-231 breast cancer cells, and uPAR expression under hypoxia in MDA-MB-231 cells depends on the phosphorylation of ERK. iNOS/NO signaling blocks uPAR expression in MDA-MB-231 cells via ERK dependent and independent pathways. The effect of iNOS/NO on HIF-1α is controversial 18, 19, 20, 34, 43.
Many studies have shown that hypoxia increases cancer cell invasion and metastasis in various tumor types via the upregulation of the uPA/uPAR signal transduction pathway 2, 3, 4, 7, 34. The uPAR gene promoter is known to possess three HIF-1α binding sites and to be activated by HIF-1α under hypoxic stress 2. However, the roles of ERK and iNOS/NO signaling in the modulation of uPAR are not well understood.
The roles that NO plays in various tumors remain controversial and prompts the question “friend or foe”. Some reports have found that NO is cytotoxic 35, 36, 37, whilst others have shown that it possesses protective properties against reactive oxygen species (ROS) 38, 39. Thus, NO probably plays a dual role that depends on its intratumoral concentration and exposure duration. Patients with iNOS positive breast carcinomas were found to have significantly poorer overall survival rates than those with iNOS negative tumors 23. Moreover, in iNOS knock out mice mammary gland tumor latency was found to be increased, suggesting that NO production in vivo promotes mammary tumor formation 31. In other models, NO was found to promote murine mammary gland tumor growth and metastasis by stimulating tumor cell migration, invasiveness and angiogenesis 22, 30, which are processes that require the sequential activations of nitric oxide synthase, guanylate cyclase and mitogen-activated protein kinase 24. However, those studies were done in normoxic states, and it was suggested that iNOS and NO have different roles in tumor invasion and metastasis and that these are determined by oxygen tension 40, 41. Moreover, most tumors develop central necrosis and hypoxic regions if they grow beyond a certain size due to inadequate vascularization 1. Study in the hypoxic state may reflect the physiologic condition of cancer. The present study utilized human MDA-MB-231 breast cancer cells under hypoxic conditions, and showed that protective roles of iNOS/NO system in human MDA-MB-231 breast cancer cells are via the down-regulation of ERK expression and consequently the inhibition of uPAR induction.
cGMP and PKG are well known second messengers iNOS/NO 24, 33, 40, 42, as may be cytochrome C oxidase in cellular mitochondria 38, 43. Graham et al. studied the influence of NO on tumor cell invasiveness in MDA-MB-231 cells under hypoxic states 3, and showed that NO reduced uPAR induction under hypoxic states in a cGMP dependent manner. However, the manner in which cGMP reduces uPAR signaling under hypoxic states has not been defined. Our study is the first study to find that iNOS/NO inhibits uPAR induction via an ERK dependent pathway in human MDA-MB-231 breast cancer cells exposed to hypoxic states, which might reduce the invasiveness and metastatic potential of breast cancer. Other studies have also found that the ERK pathway is activated under hypoxic stress 28, 29. Phosphorylation of ERK was reported to be more extensive in hypoxic human prostate cancer cells than in normoxic cells 11. A study also showed that NO inhibited the phosphorylation of ERK via cGMP mediated interference of the ras/raf pathway 44, and another that NO and cGMP mimetic drugs inhibited elastase activity in vascular smooth muscle cells 42.
The present study also shows that iNOS/NO suppressed uPAR activity in an ERK independent manner, since aminoguanidine decreased uPAR expression even in the presence of PD98059 in MDA-MB-231 cells under hypoxic stress. ROS like superoxide and hydroxyperoxynitrite are increased during hypoxia 45, and ROS have also been found to increase tumorigenesis and tumor invasion and metastasis 39, 46. The iNOS/NO signal competes with oxygen to bind cytochrome c oxidase, which could decrease ROS generation 38.
A key objective of our research is to understand changes in uPA and uPAR expression and their regulation under hypoxic conditions, since the majority of cancers grow in such an environment 1, 7, 8, 9. In the present study, uPAR expression was increased under hypoxic stress, which concurs with the study of Graham et al. which showed that hypoxia stimulates carcinoma cell invasiveness via the upregulation of urokinase receptor expression 2, 3. In our study, uPA activity (as measured by gel zymography) was modestly induced in hypoxic conditions, and uPAR was markedly induced, which suggests that an up-regulated uPA-uPAR system in hypoxia is due to both uPAR upregulation and increased uPA activity.
In endothelial cells of the porcine aorta, hypoxia activated ERK and NAD(P)H oxidase, and triggered a burst of ROS 45, and the uPA-uPAR system was also found to influence the activation of ERK, such that ERK and the uPA-uPAR system formed a positive feedback loop 8, 26, 27. Moreover, ERK has been reported to be a key regulator of tumor cell proliferation, invasion and metastasis via the ROS pathway and matrix metalloproteinase 47, 48 and the ERK pathway was found to be activated upon exposure to hypoxia 28, 29, 45. Moreover, hypoxia induced uPAR up-regulation was inhibited by the specific MEK 1/2 inhibitor, PD98059, in human prostate cancer cells 11.
These findings caused us to believe that ERK activation could be a main regulator of the uPAR system in the MDA-MB-231 human breast cancer cell line under hypoxic stress. Indeed, ERK phosphorylation was upregulated under hypoxia, and PD98059 markedly decreased uPAR expression. These results show that hypoxia can induce uPAR through ERK activation. Our results demonstrate for the first time that under hypoxic conditions the ERK signaling pathway is an upstream regulator of the uPAR system in human MDA-MB-231 breast cancer cells. In addition, ERK phosphorylation is known to be required both for HIF-1α stabilization 28, 29, 45 and the HIF-1α activation of transcriptional activity 29. Therefore, it is possible that HIF-1α has both an indirect influence on the uPA-uPAR system via the ERK signaling pathway and a direct influence on uPAR by binding its transcription region.
We suggest that the activation of ERK could result in uncontrolled tumor proliferation and invasion via the uPA-uPAR system in human MDA-MB-231 breast cancer cells under hypoxic stress. NO and iNOS have an inhibitory effect on the uPA-uPAR system by inhibiting ERK phosphorylation in MDA-MB-231 breast cancer cells exposed to hypoxic stress. Conclusively, our data provide evidence; 1) that hypoxia-induced uPAR expression in human MDA-MB-231 cells depends on ERK phosphorylation; 2) that iNOS/NO signaling inhibits uPAR induction under hypoxic conditions via ERK dependent and independent pathways.
References
Harris AL . Hypoxia—a key regulatory factor in tumor growth. Nat Rev Cancer 2002; 2:38–47.
Graham CH, Fitzpatrick TE, McCrae KR . Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood 1998; 91:3300–7.
Graham CH, Forsdike J, Fitzgerald CJ, Macdonald-Goodfellow S . Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int J Cancer 1999; 80:617–23.
Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 2003; 63:1138–43.
Fukuda R, Hirota K, Fan F, et al. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 2002; 277:38205–11.
Rofstad EK, Danielsen T . Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer 1999; 80:1697–707.
Rofstad EK, Rasmussen H, Galappathi K, et al. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase-type plasminogen activator receptor. Cancer Res 2002; 62:1847–53.
De Jaeger K, Kavanagh MC, Hill RP . Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer 2001; 84:1280–5.
Young SD, Marshall RS, Hill RP . Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A 1988; 85:9533–7.
Koong AC, Denko NC, Hudson KM, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res 2000; 60:883–7.
Lee KH, Choi EY, Hyun MS, Kim JR . Involvement of MAPK pathway in hypoxia-induced up-regulation of urokinase plasminogen activator receptor in a human prostatic cancer cell line, PC3MLN4. Exp Mol Med 2004; 36:57–64.
Ma Z, Webb DJ, Jo M, Gonias SL . Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci 2001; 114:3387–96.
de Vries TJ, van Muijen GN, Ruiter DJ . The plasminogen activation system in tumour invasion and metastasis. Pathol Res Pract 1996; 192:718–33.
Blasi F, Carmeliet P . uPAR: a versatile signaling orchestrator. Nat Rev Mol Cell Biol 2002; 3:932–43.
Schmitt M, Harbeck N, Thomssen C, et al. Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 1997; 78:285–96.
Pollanen J, Stephens RW, Vaheri A . Directed plasminogen activation at the surface of normal and malignant cells. Adv Cancer Res 1991; 57:273–328.
Melillo G, Musso T, Sica A, et al. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 1995; 182:1683–93.
Yu L, Gengaro PE, Niederberger M, et al. Nitric oxide: a mediator in rat tubular hypoxia/reoxygenation injury. Proc Natl Acad Sci U S A 1994; 91:1691–5.
Melillo G, Taylor LS, Brooks A, et al. Functional Requirement of the Hypoxia-responsive Element in the Activation of the Inducible Nitric Oxide Synthase Promoter by the Iron Chelator Desferrioxamine. J Biol Chem 1997; 272:12236–43.
Duenas-Gonzalez A, Isales CM, del Mar Abad-Hernandez M, et al. Expression of inducible nitric oxide synthase in breast cancer correlates with metastatic disease. Mod Pathol 1997; 10:645–9.
Phoa N, Epe B . Influence of nitric oxide on the generation and repair of oxidative DNA damage in mammalian cells. Carcinogenesis 2002; 23:469–75.
Vakkala M, Kahlos K, Lakari E, et al. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin Cancer Res 2000; 6:2408–16.
Loibl S, Buck A, Strank C, et al. The role of early expression of inducible nitric oxide synthase in human breast cancer. European Journal of Cancer 2005; 41:265–71.
Jadeski LC, Chakraborty C, Lala PK . Nitric oxide-mediated promotion of mammary tumour cell migration requires sequential activation of nitric oxide synthase, guanylate cyclase and mitogen-activated protein kinase. Int J Cancer 2003; 106:496–504.
Chan ED, Riches DWH . IFN-γ + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38mapk in a mouse macrophage cell line. Am J Physiol Cell Physiol 2001; 280:C441–50.
Ahmed N, Oliva K, Wang Y, et al. Proteomic profiling of proteins associated with urokinase plasminogen activator receptor in a colon cancer cell line using an antisense approach. Proteomics 2003; 3:288–98.
Aguirre Ghiso JA, Kovalski K, Ossowski L . Tumor Dormancy Induced by Downregulation of Urokinase Receptor in Human Carcinoma Involves Integrin and MAPK Signaling. J Cell Biol 1999; 147:89–104.
Minet E, Arnould T, Michel G, et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Letters 2000; 468:53–8.
Mottet D, Michel G, Renard P, et al. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J Cell Physiol 2003; 194:30–44.
Jadeski LC, Hum KO, Chakraborty C, Lala PK . Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int J Cancer 2000; 86:30–9.
Ellies LG, Fishman M, Hardison J, et al. Mammary tumor latency is increased in mice lacking the inducible nitric oxide synthase. Int J Cancer 2003; 106:1–7.
Palmer LA, Semenza GL, Stoler MH, Johns RA . Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol Lung Cell Mol Physiol 1998; 274:L212–9.
Nagao K, Takenaka S, Yamaji R, et al. Nitric oxide synthase induction, cGMP elevation, and biopterin synthesis in vascular smooth muscle cells stimulated with interleukin-1â in hypoxia. J Biochem (Tokyo) 2003; 133:501–5.
Rofstad EK, Mathiesen B, Galappathi K . Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res 2004; 64:13–8.
Radi R, Beckman J, Bush K, Freeman B . Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 1991; 266:4244–50.
Noronha-Dutra AA, Epperlein MM, Woolf N . Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett 1993; 321:59–62.
Polte T, Oberle S, Schroder H . Nitric oxide protects endothelial cells from tumor necrosis factor-alpha-mediated cytotoxicity: possible involvement of cyclic GMP. FEBS Lett 1997; 409:46–8.
Palacios-Callender M, Quintero M, Hollis VS, et al. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A 2004; 101:7630–5.
Wink D, Hanbauer I, Krishna M, et al. Nitric Oxide Protects Against Cellular Damage and Cytotoxicity From Reactive Oxygen Species. Proc Natl Acad Sci U S A 1993; 90:9813–7.
Postovit LM, Adams MA, Lash GE, et al. Oxygen-mediated regulation of tumor cell invasiveness. Involvement of a nitric oxide signaling pathway. J Biol Chem 2002; 277:35730–7.
Matthews NE, Adams MA, Maxwell LR, et al. Nitric Oxide-Mediated Regulation of Chemosensitivity in Cancer Cells. J Natl Cancer Inst 2001; 93:1879–85.
Mitani Y, Zaidi SHE, Dufourcq P, et al. Nitric oxide reduces vascular smooth muscle cell elastase activity through cGMP-mediated suppression of ERK phosphorylation and AML1B nuclear partitioning. FASEB J 2000; 14:805–14.
Mateo J, Garcia-Lecea M, Cadenas S, et al. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J 2003; 376:537–44.
Yu SM, Hung LM, Lin CC . cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation 1997; 95:1269–77.
Schafer M, Schafer C, Ewald N, et al. Role of redox signaling in the autonomous proliferative response of endothelial cells to hypoxia. Circ Res 2003; 92:1010–5.
Zhang HJ, Zhao W, Venkataraman S, et al. Activation of matrix netalloproteinase-2 by overexpression of manganese superoxide dismutase in human rreast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem 2002; 277:20919–26.
Johansson N, Ala-aho R, Uitto V, et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci 2000; 113 Pt 2:227–35.
Kim MS, Lee EJ, Kim HR, Moon A . p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res 2003; 63:5454–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, S., Lee, Y., Seo, J. et al. uPAR expression under hypoxic conditions depends on iNOS modulated ERK phosphorylation in the MDA-MB-231 breast carcinoma cell line. Cell Res 16, 75–81 (2006). https://doi.org/10.1038/sj.cr.7310010
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7310010