ABSTRACT
Auxin distribution during embryogenesis and seed germination were studied with transgenic Arabidopsis plants expressing GUS gene driven by a synthetic DR5 promoter, an auxin responsive promoter. The results showed that GUS activity is higher in ends of hypophysis and cotyledon primordia of heart-, torpedo- and cotyledon-stage embryos, leaf tip area, lateral root primordia, root apex and cotyledon of young seedlings. And GUS accumulated in root apex of the seedlings grown on auxin transport inhibitor containing media. All these suggested that above-mentioned part of the organs and tissues have a higher level of auxin, and auxin polar transport inhibitor could cause the accumulation of auxin in root apex. And auxin transport inhibitor also resulted in aberration of Arabidopsis leaf pattern formation, root gravitropism and elongation.
Similar content being viewed by others
INTRODUCTION
Auxin plays an important role in regulating cell division, elongation and differentiation, vascular tissue formation1, pollen development2 and leafy head formation 3. Auxin polar transport is believed to involve in a variety of important growth and developmental processes, including the pattern formation of embryo, leaf morphogenesis and the root gravity response4, 5, 6, 7, 8. Auxin polar transport inhibitor has been proved essential interference of auxin transport leading to pattern aberrations8. Two possible models for auxin transport and distribution were suggested to elucidate initiation of cotyledon5. It is reported that both of basipetal and acropetal auxin transport were necessary for lateral root development9, and the basipetal transport is also required for root gravitropism in Arabidopsis4. It is difficult to measure the distribution of auxin in special tissues and cells using isotope marked auxin because of low level of auxin content. The high sensitive technique of GC-MS for IAA measure needs special equipment and skilled technician8, 10. Auxin-responsive promoters (such as GH3, SAUR and DR5) provide a convenient method for studying auxin distribution in special organs or tissues11, 12, 13, 14. In addition, DR5 promoter was created by performing site-directed mutation in a natural composite auxin response element of GH3 promoter, and DR5 showed greater auxin responsiveness than GH315. It was demonstrated that the expression pattern of the GH3:GUS gene is similar to the distribution pattern of C14-IAA that is applied at the apical end gravistimulated shoot8, and auxin responsive promoter is induced specially by active auxins, but not by biologically inactive analogues, other plant hormones or environmental stress8, 10, 12. It is also reported that auxin responsive promoters have an auxin dose-dependent activation8. In this paper, we described the expression pattern of the DR5:GUS gene during embryogenesis and early germination of Arabidopsis, and reported that the auxin distribution and transport in normal embryo and seedlings could be clearly observed by using transgenic Arabidopsis plants expressing GUS gene driven by DR5. We also reported auxin distribution in seedlings and fused leaves in Arabidopsis induced by inhibition of auxin polar transport.
MATERIALS AND METHODS
Plant materials
The seeds of transgenic Arabidopsis plants expressing GUS gene driven by a synthetic promoter (DR5) consisting 7 tandem repeats of an auxin-responsive TGTCTC element and a minimal 35S promoter were kindly provided by Prof. Gulfoyle (Department of Biochemistry, University of Missouri, USA)15. Above-mentioned transgenic plants were grown on potting soil in growth chamber at 22°C under 16h photo-period light after selection with kanamycin (50 mg/L). At least 30 immature seeds were used and each experiment of distribution of auxin in embryogenesis was repeated for four to five times.
For the experiments of seed germination, the seeds were sterilized with 33%(V/V) Clorox for 15 min, washed with sterile water five times and then placed on MS medium (Sigma M-9274) supplemented with or without TIBA (2, 3, 5-triiodobenzoic acid, Sigma T-5910). Seedlings were grown under a cycle of 16h light (25°C) and 8h dark (22°C).
PCR assay
PCR assay for identification of the transgenic Arabidopsis was performed with the primer DR5 ( 5′-CCT TTT GTC TCC CTT TTG TCT C-3′ ), GUS/P1 ( 5′-GGG ATC CAT CGC AGC GTA ATG-3′ ) and GUS/P2 (5′-GCC GAC AGC AGC AGT TTC ATC-3′) designed based on DR5 promoter, 505-525 and 1048-1068 of GUS gene respectively.
Gravity response and elongation of Arabidopsis roots
The gravity response and elongation of Arabidopsis roots were measured using 3d old light grown plants. About twenty seedlings were transferred with horizontal direction on the surface of the medium in each Petri dish for 2 h, then the plates were reoriented 90o. Angles and length of roots were measured at different times.
Histochemical assays for GUS enzyme activity
Histochemical assays for GUS enzyme activity were performed as described by Jefferson16. Plant tissues were incubated in Gus assay solution (100mM sodium phosphate [pH6.8], 0.5mM potassium ferrocyanide, 0.5mM potassium ferricyanide, 10 mM) EDTA, 0.1% triton X-100, 20% methanol and 1mM 5-bromo-4-chloro-β-glucuronide) at 37°C for 8-16 h. Pigments in the tissue were removed by 95% ethanol for photographic analysis.
Gus assay and whole-mount clearing
The procedure of the technique was developed on the basis of whole-mount clearing17 and GUS assay16, which include following steps. 1) GUS assay, the immature seeds were harvested and incubated in the GUS assay solution at 37°C for 16 h. 2) Treatment of acetic anhydride, the specimen were placed in acetic anhydride solution (100 ml acetic anhydride in 5 ml 0.1 mol/L triethanalamine) for 1 h. 3) Dehydration, the specimen were passed through the following steps: H2O, 70% ethanol, 95% ethanol, and 100% ethanol, 15 min each. 4) Clearing, the specimen cleared in ethanol/methyl salicylate (1:1) mixture for 2 h followed by methyl salicylate for 2 h or overnight.
RESULTS
PCR assay was performed to confirm the transgenic Arabidopsis, and the results showed that all the kanamycin-resistant seedlings in the experiment were positive in PCR assay (data not shown). For observing the responsiveness of the transgenic Arabidopsis to auxin, histochemical GUS assay was performed with 3d -old seedlings grown on MS media without hormones. The plants were incubated in 10 mM potassium phosphate buffer (pH 6.8) containing 2 mg/L IAA for 12 h. As shown in Fig 1A and B, the GUS activity is higher in seedlings treated with IAA than that of CK.
Asymmetric distribution of GUS activity during embryognesis and seed germination of transgenic Arabidopsis
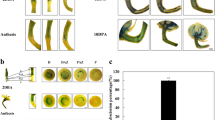
A and B showed that GUS activity is positive correlated to the exogenous IAA. The seedlings incubated in phosphate buffer and stained for GUS activity for 16h as CK (A) and the seedlings incubated in phosphate buffer containing 2mg/L IAA for 12h and then stained for GUS activity for 16h(B).
C-F showed that symmetric distribution of GUS during embryognesis of transgenic Arabidopsis. Heart-type (C), torpedo-type (D,E) and cotyledon-type embryos (F)of the transgenic Arabidopsis displayed a maximum GUS activity in ends of cotyledon primordial and hypophysis
G-I showed that the 3-day-old seedlings of the transgenic plants grown on MS medium displayed a maximum GUS activity in leaf tip area(G), lateral root primordia (H), and root apexs(I).
J and K showed that GUS activity accumulated in the root tip(K) and cotyledon(J) of the seedling grown on TIBA containing media.
L showed histochemical staining for GUS in the root of a transgenic plant after 12 h of gravity response. A larger amount of GUS activity was detected on the topside of the root that induced faster growing for gravity response
M showed GUS activity was observed in the basal end of the stem segment. Two segments cut from transgenic Arabidopsis seedling stem followed by placed horizontally on a moist filter paper for 16 h, then incubated in GUS assay solution.
N-S are some different type of trumpet-shaped-leaves (N-Q) and fused-leaves(R,S) formed in Arabidopsis seedlings grown on TIBA containing media. Q and S are distribution of GUS activity in trumpet-shaped-leaves (Q) and fused-leaves (S)
T and U showed that GUS activity accumulated in the basal end of the cut stem (T) but no GUS activity could be detect in the basal end of the cut stem in seedlings cultured in TIBA containing medium.
V showed the distribution of GUS activity during lateral root development with a 9- day-old transgenic Arabidopsis grown on MS medium.
W showed that lateral root development initiation was inhibited obviously by TIBA with a 9-day-old transgenic
In order to visualize auxin distribution during embryogenesis, the immature seeds were stained with GUS assay solution and then cleared by methyl salicylate. All the heart, torpedo and cotyledon embryos of the transgenic Arabidopsis displayed a maximum of GUS activity in ends of cotyledon primordia and hypophysis (Fig1C-F), but the GUS activity is too low to be detected in globular embryo and younger ones.
The young seedlings of the transgenic plants showed a maximum GUS activity in leaf tip area (Fig 1G), lateral root primordia (Fig 1H) and root apex (Fig 1I). And GUS activity accumulated in cotyledon (Fig 1J) and root apex (Fig 1K) of the seedling grown on auxin polar transport inhibitor containing media. The experiments here also showed that GUS activity accumulated in the basal end of the cut stem (Fig 1M, T) but no GUS activity was detected in the basal end of the cut stem in seedlings cultured on auxin polar transport inhibitor containing medium (Fig 1U). Trumpet-shaped- and fused- leaves could form in Arabidopsis seedlings grown on auxin polar transport inhibitor containing media (Fig 1N-S). Lateral root initiation was inhibited obviously by auxin polar transport inhibitor (Fig 1V and W). Fig 2 and 3 showed that TIBA inhibited elongation of roots and gravity response.
DISCUSSION
The progress of molecular biology and genetics has made the studies on complex subject, biological function of auxin and its polar transport at molecular and cellular aspects. A serial studies of development in auxin research field have been obtained by using the model plant, Arabidopsis, such as the gene cloning of auxin binding protein, auxin efflux carrier and auxin responsive genes14,18. A major limitation of plant biology is its inability to accurately assess the auxin levels13. The expression of reported genes, such as GUS gene driven by auxin responsive promoter might make us more convenient to study the relationship between distribution of auxin in plant cells/tissues and plant growth and development. And this sort of example is increasing now11, 12, 13, 14. We have used an indirect method to assess the auxin responsiveness of Arabidopsis embryo and young seedlings. The experiments used the synthetic auxin responsive promoter, DR5.
DR5 promoter responses rapidly to active auxin between 10−8 and 10−5, and remains at high level up to 10−4 mol/L11. It has demonstrated that DR5 is the best tool to visualize response to active auxins at cellular resolution level7. The present results showed a higher level auxin in root apex and ends of cotyledon primordia from heart to mature embryo. In young seedlings auxin may accumulate in leaf tip area, lateral root primordia and root apex. The auxin content in globular embryos and younger ones was relatively low. PIN1, a efflux carrier was accumulated in the four innermost cells in the lower part of the mid-globular embryo (the future root apex), and gradually narrowed down to vascular precursor cells, both in the developing cotyledon and in the embryo axis19. Therefore the asymmetric distribution of auxin in developing embryos might be contributed to asymmetric location of PIN1 protein in auxin polar transport.
Transgenic plants expressing auxin responsive promoter provides a convenient method for the study of auxin transport. Fig 1M, T and U showed that auxin accumulated in biological basal end of excised stem because of auxin transport. This result provided a new exemplification for that auxin polar transport inhibitors could trully interfere auxin transport in plants8. The present results also showed that auxin polar transport inhibitor caused accumulation of auxin in the root apex of the seedling, and it also inhibited initiation of lateral root and elongation of roots. These results supported the presumption that auxin transport inhibitors caused accumulation of auxin in the root apex while reducing levels in basal tissues critical for initiation of lateral root 9 and provided additional support for that auxin synthesized in root apex first and then transported to other part of the root. The present results also showed trumpet-shaped- and fused- leaves could form in Arabidopsis seedlings grown on auxin polar transport inhibitor containing media (Fig 1N-S), that provided a new exemplification that auxin polar transport is required in foliar leaf pattern formation6.
References
Davies PJ . Plant Hormones, Dordrecht. The Netherlands: Kluwer Academic Publishers, 1995.
Ni Di-an, Yu Xiao-hong, Wang Ling-jian, Xu Zhi-hong . Aberrant development of pollen in transgenic tobacco expressing bacterial iaaM gene driven by pollen- and tapetum- specific promoters. Acta Experimental Sinica, 2002; (in press).
He YK, Xue WX, Sun YD, Yu XH, Liu PL . Leafy head formation of the progenies of transgenic plants of Chinese cabbage with exogenous auxin genes. Cell Research, 2000; 10(2):151–602.
Rashotte AM, Brady SR, Reed RC, Ante SJ and Muday GK . Basipetal auxin transport is required for gravitropism in root of Arabidopsis. Plant Physiology 2000; 122:481–90.
Liu Chun-ming, Xu Zhi-hong, Chua Nam-hai . Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993; 5:621–30.
Ni Di-an, Wang ling-jian, Xu Zhi-hong et al. Foliar modifications induced by inhibition of auxin polar transport. Cell Research 1999; 9(1):27–35.
Xu Zhi-hong, Ni Di-an . Modifications of leaf morphogenesis induced by inhibition of auxin polar transport. In: Altman A. et al.(eds.), Plant Biotechnology and In Vitro Biology in the 21th Century, Kluwer Academic Publ., Dordrecht 1999; pp.97-9(1):27–35.
Li Y, Wu YH, Hagen G and Guilfoyle TJ . Expression of the auxin-inducible GH3 promoter/GUS fusion gene as a useful molecular marker for auxin physiology. Plant Cell Physiol 1999; 40(7):675–82.
Casimiro I, Marchant A, Dhooge S et al. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell 2001; 13:843–52.
Fischer-lglesias C, Sundberg B, Neuhaus G et al. Auxin distribution and transport during embryonic pattern formation in wheat. The Plant J 2001; 26:115–29.
Sabatini S, Bels D, Wolkenfelt H, Murfett J et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999; 99:463–72.
Li Y, Hagen G, Guilfoyle TJ . An auxin-response promoter is differential induced by auxin gradients during tropisms. The Plant Cell 1991; 3:1167–75.
Mathesius U, Schlaman HRM, Spaink HP et al. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligsaccharies. The Plant J 1998; 14(1):23–34.
Ni Di-an and Xu Zhi-hong . Auxin, auxin biosynthesis and its transport. Plant Physiology Communications 2001; 37(4):346–52.
Ulmasov T . Murfett J . Hagen G, Gulfoyle TJ . Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 1997; 9:1963–71.
Jefferson RA, Kavanagh TA, Bevan MW . GUS fusions:b-glucuronidase as a sensitive and veratile gene fusion maker in higher plants. EMBO J 1987; 6:3901–7.
Zhu ZP, Shen RJ, Tang XH . Polyembryo in rice and its germination. Chinese Journal of Botany 1990; 2(1):39–44.
Ni Wei-min, Chen Xiao-ya, Xu Zhi-hong, Xue hong-wei . Advances in study of polar auxin transport. Acta Botanica Sinica 2000; 42(3):221–8.
Steinmann T, Gnldner N, Grebe M et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM AFR GEF. Science 1999; 286:316–8.
Acknowledgements
The authors want to thank Prof. Zhu Zhi-ping and Mr.Yang Zhi-xing (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences) for their enlightening discussion, and Prof. Gulfoyle (Department of Biochemistry, University of Missouri, USA) for his kindly providing seeds of transgenic Arabidopsis. This work was supported by National Natural Science Foundation of China (39770079).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
NI, D., WANG, L., DING, C. et al. Auxin distribution and transport during embryogenesis and seed germination of Arabidopsis. Cell Res 11, 273–278 (2001). https://doi.org/10.1038/sj.cr.7290096
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290096
Keywords
This article is cited by
-
The Microphenotron: a robotic miniaturized plant phenotyping platform with diverse applications in chemical biology
Plant Methods (2017)
-
microRNAs participate in gene expression regulation and phytohormone cross-talk in barley embryo during seed development and germination
BMC Plant Biology (2017)
-
Proteomic Analysis of the Protein Expression Profile in the Mature Nigella sativa (Black Seed)
Applied Biochemistry and Biotechnology (2016)
-
The Arabidopsis E3 ubiquitin ligase HOS1 contributes to auxin biosynthesis in the control of hypocotyl elongation
Plant Growth Regulation (2015)
-
The bHLH transcription factor SPATULA regulates root growth by controlling the size of the root meristem
BMC Plant Biology (2013)