Abstract
Objective:
To evaluate blood gases and ventilatory parameters before and after two doses of surfactant in premature infants with respiratory decompensation after recovery from primary respiratory distress syndrome (RDS).
Study Design:
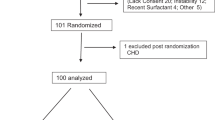
This prospective pilot study enrolled infant's ⩾500 g birth weight, from 7 days to 3 months of age, with a secondary respiratory decompensation lasting at least 4 h prior to study entry. Infants received two doses of surfactant, 12 h apart.
Result:
A total of 20 neonates qualified for secondary surfactant administration. PCO2 (P<0.001); pH (P<0.001); mean airway pressure (P<0.05); FiO2 (P<0.05); modified ventilatory indices (P<0.004) and respiratory severity scores (P<0.001) improved significantly at both 12 and 24 h after surfactant administration.
Conclusion:
Secondary surfactant administration may be effective in reducing short-term ventilatory requirements in neonates who have a respiratory decompensation after recovery from initial RDS. Randomized controlled trials are needed to confirm these preliminary findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hamilton B, Ventura S, Martin J, Sutton P . Preliminary births for 2004 National Center for Health Statistics. Health E-Stats 2004 [cited 2006]; Available on http://www.cdc.gov/nchs/products/pubs/pubd/hestats/prelim_births/prelim_births04.htm.
Jobe A, Bancalarie E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729.
Merrill J, Ballard R, Cnann A, Hibbs A, Godinez R, Godinez M et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr Res 2004; 56 (6): 918–926.
Kaneko M, Watanabe J, Ueno E . Surfactant lavage and replacement in meconium aspiration syndrome with pulmonary hemorrhage. J Perinat Med 2001; 29: 351–356.
Harms K, Herting E . Successful surfactant replacement therapy in two infants with ARDS due to chlamydial pneumonia. Respiration 1994; 61: 348–352.
Pandit P, Dunn M, Colucci E . Surfactant therapy in neonates with respiratory deterioration due to pulmonary hemorrhage. Pediatrics 1995; 95 (1): 32–36.
Amizuka T, Shimizu H, Niida Y, Ogawa Y . Surfactant therapy in neonates with respiratory failure due to haemorrhagic pulmonary oedema. Eur J Pediatr 2003; 162: 697–702.
Pandit P, O’Brien K, Asztalos E, Colucci E, Dunn M . Outcome following pulmonary haemorrhage in very low birthweight neonates treated with surfactant. Arch Dis Child Fetal Neonatal Ed 1999; 81: 40–44.
Dargaville P, Mills J, Soll R . Therapeutic lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev 2002; 4: 1–5.
Herting E, Moller O, Schiffmann J, Robertson B . Surfactant improves oxygenation in infants and children with pneumonia and acute respiratory distress syndrome. Acta Paediatr 2002; 91: 1174–1178.
Wiswell TE, Knight G, Finer N, Donn S, Desai H, Walsh W et al. A multicentered, randomized, controlled trial comparing Surfaxin (Lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics 2002; 109: 1081–1087.
Herting E, Gefeller O, Land M, van Sonderen L, Harms K, Robertson B, Members of the Collaborative European Multicenter Study Group. Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics 2000; 106 (5): 957–965.
Tibby S, Hatherill M, Wright S, Wilson P, Postle A, Murdoch L . Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2000; 162 (4): 1251–1256.
Pandit PB, Dunn MS, Kelly EN, Perlman M . Surfactant replacement in neonates with early chronic lung disease. Pediatrics 1995; 95: 851–854.
Merrill J, Ballard P, Hibbs A, Godinez R, Godinez M, Luan X et al. Booster surfactant therapy beyond the first week of life in ventilated extremely low gestation age infants. Pediatr Res 2004; 56: 918–926.
Katz L, Klein J . Repeat surfactant therapy for postsurfactant slump. J Perinatol 2006; 26: 414–422.
Bissinger R, Carlson C, Hulsey T, Eicher D . Secondary surfactant deficiency in neonates. J Perinatol 2004; 24: 663–666.
Notter RH, Egan EA, Kwong MS, Holm BA, Shapire DL . Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: effects of dose, dispersion technique and gestational age. Pediatr Res 1985; 19: 569–577.
Ilce Z, Guney C, Eray N, Ilikkan B, Celayir S . The role of modified ventilatory index in defining the prognosis in surgical and non-surgical pediatric patients. Internet J Pulm Med 2005; 5 (1): 1–8.
Rivera R, Butt W, Shann F . Predictors of mortality in children with respiratory failure: Possible indications for ECMO. Anaesth Int 1990; 18: 385–389.
Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D . Congenital diaphragmatic hernia—a tale of two cities: the Toronto experience. J Pediatr Surg 1997; 32 (3): 395–400.
Sakura Y, Azarow K, Cutz E, Messineo A, Pearl R, Bohn D . Pulmonary barotrauma in congenital diaphragmatic hernia: a clinicopathological correlation. J Pediatr Surg 1999; 34 (12): 1813–1817.
Keshen T, Gursoy M, Shew S, Smith E, Miller R, Wearden M et al. Does extracorporeal membrane oxygenation benefit neonates with congenital diaphragmatic hernia? Application of a predictive equation. J Pediatr Surg 1997; 32 (6): 818–822.
Lewis J, Veldhuizen R . Factors influencing efficacy of exogenous surfactant in acute lung injury. Biol Neonate 1995; 67 (Suppl 1): 48–60.
Enhorning G, Shennan A, Possmayer F, Dunn M, Chen C, Milligan J . Prevention of neonatal respiratory distress syndrome by tracheal administration of surfactant: a randomized clinical trial. Pediatrics 1985; 76: 145–153.
Wilson D, Zaritsky A, Bauman LA, Dockery K, James RL, Conrad D et al. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Crit Care Med 1999; 27 (1): 188–195.
Mulrooney N, Jobe A, Ikegami M . Lung inflammatory responses to intratracheal interleukin-1alpha in ventilated preterm lambs. Pediatr Res 2004; 55 (4): 682–687.
Acknowledgements
We thank the Neonatal Nurse Practitioner Team and the Respiratory Therapists at MUSC for their support and assistance during this study. We especially thank three NNPs, Mary Kay Colliton, Ashley Klumb and Margaret Conway-Orgel, who participated on the research team and our research nurse, Deanna Fanning. This study was partly funded by ROSS Pharmaceuticals and Dey Laboratories. ROSS Pharmaceuticals provided Survanta for study patients at no cost. Dey Laboratories provided an educational grant to cover the cost of the Curosurf. No other funding was provided for this study and none of these companies had involvement in the research or access to the outcome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bissinger, R., Carlson, C., Michel, Y. et al. Secondary surfactant administration in neonates with respiratory decompensation. J Perinatol 28, 192–198 (2008). https://doi.org/10.1038/sj.jp.7211909
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211909
Keywords
This article is cited by
-
Organ dysfunction and mortality in preterm neonates with late-onset bloodstream infection
Pediatric Research (2023)
-
Late administration of surfactant replacement therapy increases surfactant protein-B content: a randomized pilot study
Pediatric Research (2012)