Abstract
Purpose
To determine the effects of pericytes and angiopoietin-1 on the expression of occludin and zonula occludens-1 (ZO-1) in retinal endothelial cells (ECs) under both normoxic and hypoxic conditions.
Methods
Rat primary retinal microvascular ECs were cultured under normoxia or hypoxia in either absence or presence of pericytes conditioned medium (PCM). PCM was pretreated with or without angiopoietin-1 neutralizing antibody. Immuofluorescent staining, Western blot and RT-PCR were used to detect the alterations of occludin and ZO-1 expression.
Results
Under normoxia, PCM strengthened occludin and ZO-1 immunofluorescent staining at cytomembrane as well as increased their expression at both protein and mRNA level. When pretreated with angiopoietin-1 neutralizing antibody, occludin upregulation induced by PCM was significantly blocked at protein level (62%) and mRNA level (34%). Under hypoxia, the continuity of occludin and ZO-1 staining at cell boundaries was disrupted consistent with a decrease of their protein level by 31 and 27%, respectively. Also occludin and ZO-1 mRNA level decreased by 46 and 57%, respectively. PCM was observed to partially increase expression of occludin at protein and mRNA level. Angiopoietin-1 antibody slightly inhibited (16%) PCM induced occludin mRNA increase under hypoxia.
Conclusion
Pericytes improved the integrity of endothelial barrier through inducing occludin and ZO-1 expression at protein and mRNA level under normoxia. Under hypoxia, pericytes could partially reverse occludin decrease. These protecting effects of pericytes on endothelial barrier were at least in part mediated by angiopoietin-1.
Similar content being viewed by others
Introduction
Retinal neovascularization is a common sequela of widespread retinal diseases, such as diabetic retinopathy, retinopathy of prematurity and retinal vein occlusion. Immature vessels in these diseases are characterized by leakage, and one suggested mechanism behind which is the damage of endothelial barrier. Retinal microvascular endothelial cells (RMECs) differ from other non-barrier endothelial cells (ECs) in the highly specialized tight junction (TJ) complexes. Occludin was the first transmembrane protein discovered and located in TJs. Members of the claudin family and the junctional adhesion molecules (JAM) were discovered subsequently.1, 2 The transmembrane protein binds to members of the zonula occludens protein family (ZO-1, ZO-2, and ZO-3), which are connected to the cytoskeleton proteins such as actin. TJs are mainly located in blood–brain barrier (BBB), and have recently been detected in retinal vascular ECs.3 Current knowledge suggests that occludin and ZO-1 are key TJ proteins in retinal TJs. Their importance could be corroborated by their downregulation in TJs disruption4 and their increased expression in improved barrier function.5
Current studies on ECs barrier in CNS generally focus on the effects of astrocytes. Although astrocytes are able to strengthen the expression of certain BBB properties6 and helped to improve the integrity of ECs barrier, they could only add limited advantages to the physical integrity of BBB.7 Whereas, pericytes, another closely related cell type which intimately embracing capillary endothelium, have always been overlooked in the endothelia cell barrier, especially in pathological conditions such as hypoxia.
Pericytes play a key role in normal structure and function of mircovessels. Pericytes, which surround ECs, are responsible for the equilibrium of the ECs microenvironment.8 Recruitment and coverage of pericytes in microvessels are key processes in normal vascular development, maturation and maintenance. In pericyte deficiency models of newborn mice, grave retinal vascular leakage, oedema, and haemorrhage emerged,9 which could be possibly induced by redistribution of certain junctional proteins such as VE-cadherin and occludin.10 Furthermore, pericyte coverage rates of the abluminal endothelial surface vary extensively among different tissues and the highest pericyte density has been described in the retina as 50%,11 which may reflect its importance to retinal endothelial barrier.
However, little is known about the mechanisms of pericyte function in retinal endothelial barrier. Angiopoietin-1 (Ang-1), derived from pericytes, is known to be an anti-permeability factor in vascular system. Angiopoietin-1 has also been proven to have a protective effect on blood retinal barrier (BRB) via inhibiting VEGF induced retinal vascular leakage.12 The mechanism of anti-permeability effect of angiopoietin-1 lies in its downregulation of VE-cadherin and PECAM-1 phosphorylation, which strengthens the cell adherens junctions.13 Whereas, whether angiopoietin-1 could prevent the retinal vascular leakage by induction of TJs' expression is not yet known.
Hypoxia is currently considered to be a primary stimulus in retinal neovascularization and the BRB disruption. RMECs represent a specific population of ECs characterized by a high number of TJs and low pinocytotic rate. Recent studies indicate that hypoxia-induced disruption of the BBB is partially due to loosening in TJs, resulting in alterations of paracellular permeability pathway.14 Hypoxia could activate TGF-beta, which would subsequently induces MMP-9 expression in bovine RMECs. The latter would increase the permeability of RMECs monolayers and reduce the occludin level.15
This study was performed to gain insight into pericytes' paracrine effect on RMECs paracellular permeability in TJs under normoxic and hypoxic conditions. Furthermore, the role of Ang-1 was also investigated to clarify the possible signal pathway for pericytes effects.
Materials and methods
Isolation and identification of RMECs and pericytes
Primary RMECs from fresh Wistar rat retina were isolated as described previously16 with a modified purification method of using anti-CD31 antibody labelled Dynabeads (Dynal Biotech, USA). Briefly, retinae were removed aseptically, then minced and filtered through a 53 μm nylon mesh. The filtrate was discarded and the mesh rinsed in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) to suspend the tissue and then centrifuged. The pellets were digested by 0.1% collagenase type I for 30 min at 37°C with agitation. The cellular digests were filtered through a 30 μm nylon mesh and then centrifuged. The pellets were suspended in DMEM containing 10% foetal bovine serum (FBS) and incubated with CD31 antibody labelled Dynabeads for 30 min at 4°C. After affinity binding, Dynabeads were washed six times with DMEM/10% FBS. Pericytes in eluant were centrifuged and suspended in pericytes growth medium followed by seeding onto culture flask.17 Dynabeads binding RMECs were seeded onto rat tail collagen type I-coated tissue culture flask.
RMECs grew in DMEM supplemented with 10% FBS, 20 mM sodium bicarbonate, 100 U/ml benzylpenicillin sodium and 100 U/ml streptomycin sulphate, freshly added heparin at 55 U/ml and EC growth supplement 100 μg/ml (ECGS) (Sigma, USA) (culture medium-A). The culture medium-B for pericytes consisted of culture medium-A without heparin and ECGS, 20% FBS instead.
RMECs were identified by polyclonal rabbit VIII antibody (1 : 100, Santa Cruz, USA) staining and monoclonal mouse anti-rat CD31 antibody (1 : 100, Chemicon, USA) plus Cy3-conjugated second goat anti-mouse antibody. Pericytes were identified by polyclonal rabbit anti-PDGFR-β antibody (1 : 100, Santa Cruz) and mouse anti-rat desmin (1 : 100, Dako, USA) with FITC or Cy3-conjugated second antibody, respectively. Cells between passages 2 and 6 were used in this experiment.
Preparation of various treatments
Pericytes conditioned medium (PCM) was prepared as follow: when the cultured pericytes reached confluence, the medium was replaced with DMEM containing 1% FBS, and incubated at 37°C for 24 h. The conditioned medium was harvested, filtrated with a sterile 0.22 μm filter and stored frozen at −20°C until use. Control CM (CCM) was prepared in the similar way from the media but without cells. Briefly, DMEM with 1% FBS, but without pericytes, was incubated at 37°C for 24 h, then harvested and frozen at −20°C after filtrated.
Hypoxic conditions were achieved by adding CoCl2 (200 μM) to medium to mimic hypoxia.18 RMECs were cultured for 24 h under hypoxic condition.
Angiopoietin-1 inhibition study: PCM was pre-treated with 1 μg/ml antibody against angiopoietin-1 (Chemicon, USA) or normal rabbit IgG (control) for 16 h.
Experimental groups
RMECs under various treatment were divided into six groups as follows: RMECs cultured in control CM under normoxia (EN), RMECs cultured in control CM under hypoxia (EH), RMECs cultured in PCM under normxia (PEN), RMECs cultured in PCM under hypoxia (PEH), PEN pretreated with Ang-1 antibody (PENA) and PEH pretreated with Ang-1 antibody (PEHA).
Immunofluorescence
Immunofluorescent staining of occludin and ZO-1 was performed as follows: RMECs grown on slides were washed once with PBS and fixed by immersion in 4% paraformaldehyde in PBS for 30 min, then permeabilized in PBS with 0.1% Triton X-100 for 10 min at room temperature. Cells were blocked for 1 h with PBS containing 10% horse serum and 0.1% Tween 20. Permeabilized cells were incubated overnight at 4°C with primary antibodies. Rabbit anti-occludin antibodies (Zymed, South San Francisco, CA, USA) were combined with PBS in a 1 : 100 dilution or rabbit anti-ZO-1 antibodies (Santa Cruz, USA) at a 1 : 100 dilution. Cells were washed with PBS-Tween (PBS with 0.1% Tween 20) and stained with FITC-labelled goat anti-rabbit antibodies, or Cy3-conjugated goat anti-rabbit antibodies (Sigma, USA) at a 1 : 100 dilution respectively in PBS-Tween for 1 h at 37°C. Cells then were rinsed three times in PBS-Tween, mounted in 50% glycerol in PBS, and examined with a laser scanning confocal microscope (LSCM, MRC1024, Bio-Rad, USA).
Western blot analysis
An occludin or ZO-1 enriched extract were prepared according to the method of Brankin.19 Protein concentration was determined by BCA protein assay and equal amounts of protein were loaded onto 6–10% SDS-PAGE gels. Protein samples were electrotransferred to nitrocellulose membranes with 250 mA at room temperature for 90 min. The membranes were then blocked using 5% nonfat milk-Tris-buffered saline (20 mM Tris base, 137 mM NaCl, pH 7.6) with 0.1% Tween 20 for 4 h at room temperature and were then incubated overnight at 4°C with occludin (1 : 2000), ZO-1 (1 : 1000) and β-actin (1 : 1000) antibody in PBS-0.5% BSA. The membranes were washed three times with 5% nonfat milk-Tris-buffered saline buffer, and incubated with the HRP conjugated goat-anti-rabbit (DAKO, 1 : 2000) antibody for 60 min at room temperature. Blots were developed using the enhanced chemiluminescence method and protein bands were visualized on X-ray film. Semiquantification of the protein was carried out with the use of Scion image software and the results are reported as percentages of controls.
RT-PCR analysis
Total RNA was extracted from RMECs using an RNeasy kit (Qiagen, Tokyo, Japan) according to the manufacturer's protocol. Primers for occludin (GenBank Accession No. AB016425) were: sense 5′-CTGTCTATGCTCGTCATCG-3′ and antisense 5′-CATTCCCGATCTAATGACGC-3′, with an expected product of 294 bp. Primers for ZO-1 (GenBank Accession No. XM068518) were: sense 5′-GCCTCTGCAGTTAAGCAT-3′ and antisense 5′-AAGAGCTGGCTGTTTTAA-3′, with an expected product of 249 bp. Primers for β-actin (GenBank AA874855) were: sense 5′-TTCCACACACACCAGCTTCG-3′ and antisense 5′-GGGGTGGTGTGGAGATTTAG-3′, with an expected product of 366 bp.
RT-PCR was carried out using a reaction system (TakaRa one-step RNA PCR kit, Japan) as: total sample RNA 1 μg, specific primers 1 μl, 10 × Tris-HCl buffer 5 μl, MgCl2 (25 mM) 10 μl, 10 mM dNTP Mixture 5 μl, 40 U/μl RNase inhibitor 1 μl, 5 U/μl AMV reverse transcriptase 1 μl and 5 U/μl AMV-Optimized Taq 1 μl in a final volume of 50 μl.
Reverse transcription was carried out at 50°C for 30 min and inactivated at 94°C for 2 min. The parameters for PCR were as follows: occludin: denaturation at 94°C for 1 min, annealing at 62°C for 2 min, and extension at 72°C for 3 min for a total of 23 cycles, followed by a 15 min extension at 72°C. ZO-1: denaturation at 94°C for 1 min, annealing at 56°C for 2 min, and extension at 72°C for 2 min for a total of 22 cycles, followed by a 10 min extension at 72°C.20 β-Actin: 28 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 60 s, and ended with elongation at 72°C for 5 min. PCR-amplified products were visualized in a 1.5% agarose gel stained with ethidium bromide. A DNA ladder (DL2000 Marker TakaRa, Japan) was loaded on each gel. Quantification of PCR products was performed using the UVP Gel Documentation System GDS7500. The density of individual lanes was normalized to the density of the PCR amplified internal control β-actin.
Statistical analysis
For all assays, three or more separate experiments were repeated. Mean±SD from several experiments were calculated. One-way ANOVA was used to assess statistical significance of differences among means of two or more than two groups. The level of statistical significance was set at a P-value of 0.05.
Results
Isolation and identification of rat RMECs and pericytes
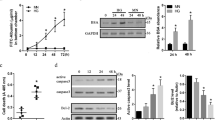
Highly pure and active RMECs were successfully harvested by CD31coated Dynabeads (Figure 1a). RMECs clones formed and reached confluence during the following 4–5 weeks (Figure 1b). Later, RMECs were identified by immunocytochemical staining using anti-Factor VIII antibody and anti-CD31 antibody (Figures 1c and d). Rat retinal pericytes were isolated by selective culture condition and could passage continuously. Pericytes showed irregular shapes with thick filament in the cytoplasm and overlapping growth pattern without contact inhibition (Figure 1e). Pericytes were identified by immunofluorescent staining with PDGFR-β and desmin antibody (Figure 1f) under a laser scanning confocal microscope.
Morphology and identification of rat RMECs and rat retinal microvascular pericytes. (a) RMECs (black arrow) clump isolated by Dynabeads (white arrow) 24 h after primary culture with phase-contrast microscopy (× 200). (b) RMECs in a confluent monolayer with phase-contrast microscopy (× 100). (c) Immunocytochemical staining with anti-Factor VIII antibody to RMECs (DAB × 100). (d) Immunofluorescence staining with anti-CD31 anibody to RMECs (Cy3 × 100). (e) Photograph of passaged rat pericytes, showing non-contact-inhibited growth with irregular shapes and fibre bundles (× 100). (f) Merge of PDGFR-β and desmin in pericytes with LSCM (× 200).
Change in expression and distribution of TJ proteins
Maintenance of TJs during RMEC culture
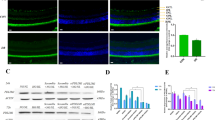
After 8-day culture, RMECs reached confluence and acquired the elongated shape. Occludin largely accumulated along the cellular periphery in continuous lines, with comparatively weak staining in cytoplasm (Figure 2a). Distribution pattern of ZO-1 is similar to occludin, but less continuous along the cell boundary (Figure 3a).
Variation of TJ distribution after hypoxia
CoCl2 was added to the culture media at day 7. After 24 h hypoxic incubation, occludin and ZO-1 staining were clustered in patchy aggregates in cytoplasm and continuous staining along cell boundaries was scarcely observed (Figures 2b and 3b).
TJ proteins alteration after cultured in PCM and Ang-1 antibodies
When RMECs were cultured in PCM under normoxia, lines of occludin and ZO-1 along the cell boundaries were observed to be more continuous than in EN group. In line with this, the expression of both proteins appeared to decrease in cytoplasm (Figures 2c and 3c). When cultured in PCM under hypoxia (PEH group), distribution change of occludin and ZO-1 was partially reversed, but could not reach their normal state (Figures 2d and 3d).
After pretreatment with Ang-1 antibody under normoxia, TJ proteins strengthening effects by PCM were mostly blocked (Figures 2e and 3e). When same treatments were applied under hypoxia (PEH group), staining of occludin and ZO-1 at cell boundaries was only slightly stronger than in EH group (Figures 2f and 3f).
Western blot analysis of TJs
Occludin and ZO-1 antibodies recognized 65 and 220 kDa polypeptides, respectively (Figure 4). Densitometry analysis of Western blots (Figure 4a and b) revealed that hypoxia induced a 31% (31.8±4.6%; P<0.05) decrease in occludin and a 27% (27.5±4.1%; P<0.05) decrease in ZO-1 compared to EN group.
Proteins expression of occludin and ZO-1 was detected by Western blot. Representative Western blots for occludin and ZO-1 were shown. Results were normalized by β-actin protein loading and converted to percent of control (EN). The quantifications were shown on the graphs below: (a) Occludin expression (b) ZO-1 expression. Data are means±SD from n=3 experiments (*P<0.05).
Occludin and ZO-1 density in PEN group were increased significantly by 1.96-fold (196.3±22.5%; P<0.05) and 1.21-fold (121.6±14.5%; P<0.05) of that in EN group, respectively. When cultured in PCM under hypoxia, occludin expression increased slightly by 1.15-fold (115.9±12.5%; P<0.05) compared to EH group and no increasing of ZO-1 was observed.
When pretreated with Ang-1 antibody under normoxia, PCM induction of occludin was reduced by 62% (62.4±8.5%; P<0.05). Whereas, ZO-1 was not affected by the same pretreatment. Under hypoxia, Ang-1 antibody pretreatment failed to inhibit PCM's effect on occludin.
RT-PCR analysis of TJs
The influence of PCM on TJs at mRNA level was further examined using RT-PCR (Figure 5). After 24 h hypoxia, occludin and ZO-1 mRNA levels were 46% (46.4±5.5%; P<0.05) and 57% (57.3±7.5%; P<0.05) lower compared to EN group, respectively.
After PCM culture under normoxia, occludin mRNA level rose by 81% (81.2±10.3%; P<0.05), while ZO-1 mRNA expression only rose slightly by 12% (12.3±2.6%; P<0.05) in comparison to EN group. Under hypoxia with PCM cultrue, occludin mRNA expression rose by 43% (43.6±6.5%; P<0.05). And no increase in ZO-1 mRNA expression was observed.
After pretreatment with Ang-1 antibodies under normoxia, occludin mRNA expression decreased by 34% (34.3±4.2%; P<0.05) compared to PEN group. Under hypoxia, occludin mRNA decreased about 16% (16.7±2.4%; P<0.05). Still, Ang-1 antibodies had no effects on ZO-1 mRNA expression.
Discussion
Early passage RMECs maintained their in vivo characteristics such as TJ expression and low TEER values, which contribute to the function of BRB.21 Therefore, these cells are a promising candidate to allow insights into BRB. Primary cultured rat RMECs were utilized in our experiment for their identified immunocytochemical characteristics and considerable expression in TJs.
Pericytes have been demonstrated to play an important role in inducing ECs to express endothelial barrier phenotype. Furthermore, PCM can enhance BBB properties of brain microvascular ECs.22 But little is known about the details of pericytes' effects on TJs especially in retinal ECs. Occludin is highly expressed in barrier endothelia and acts as a key TJ protein whose level dictates tissue barrier properties.23 Increased expression of occludin has been found to correlate with improved barrier function through elevating TEER in several cell lines.24 Although not a featured protein of TJs, ZO-1 accurately reflects the pathological changes of BBB, which made it a valuable marker of endothelial barrier.25 Pericytes may induce and maintain the properties, including the integration of TJs, of the BBB through paracrine and cell-to-cell contact. In this experiment, we attempted to determine pericytes' paracrine effect on RMECs. PCM was used to detect the possible involved soluble factors. Our results showed that under normoxia, PCM strengthened the continuity of occludin and ZO-1 at cell boundaries and meanwhile decreased both proteins in cytoplasm. The relocation of both proteins suggested that PCM could strengthen TJs formation. In addition to trigger proteins relocation, PCM could also increase occludin and ZO-1 expression at the protein level as well as at the mRNA level. PCM's effects on occludin and ZO-1 follow same trends, indicating the amount of proteins in cells was partially regulated via transcription. These results indicated pericytes may improve function of retinal endothelial barrier through soluble factors.
Previous in vivo DR model showed that Ang-1 was a powerful anti-permeability factor in BRB via inhibiting VEGF, ICAM-1, and NO.26 Our data showed that Ang-1 neutralizing antibody pretreatment prominently blocked PCM's TJ-strengthening effect through downregulating occludin. As reported previously, Ang-1, derived from pericytes, performed its total effects through Tie2 receptor on ECs.27, 28 Our results suggested that Ang-1 could partially mediate the pericytes' effect on RMECs TJs. Although Ang-1 could block occludin expression to a great extent, the blockage is not complete. Furthermore, ZO-1 protein level, enhanced in PCM, was unaffected, which implied that other factors were involved in pericyte function. Latest research demonstrated that pericytes could strengthen BBB, which was partly mediated through continuously producing TGF-β1.29 As another soluble factor derived from pericyte, bFGF was also found to tighten the intercellular junctions.30 Ang-1, combined with TGF-β1 and bFGF, may play an important role in pericytes' TJs strengthening effect. Further studies, however, are still needed to clarify the interaction between these factors and their relevant signal pathways.
It was observed in our study that hypoxia decreased occludin and ZO-1 staining along cells boundaries and increased both staining in cytoplasm, which is consistent with previous results in porcine brain microvessel ECs.31 The possible mechanism may be the selective phosphorylation of key components of occludin and ZO-1, as protein phosphorylation plays a key role in the regulation of protein function.32 Under hypoxia, production of ATP is markedly reduced, inhibiting the activity of phosphorylation. Protein kinase C (PKC) activation under hypoxia may also contribute to decreased phosphorylation of occludin on threonine.33 Lack of phosphorylation then inevitably reduce the active form of occludin and ZO-1, leading to the decreased secretion of both proteins to cytomembrane. The relocation of both proteins caused by hypoxia initiated occludin-ZO-1-actin chain break, ultimately leads to alteration of cell conformation and endothelial barrier disruption.
Besides protein relocation, hypoxia was shown to downregulate occludin and ZO-1 in protein level in our study. The mechanism of this downregulate is under extensive investigation. As known, the chief protein degradation pathway in the cytoplasm is the constitutive proteasome pathway. The complex was then recognized by proteasomes and degraded. Traweger recently showed that occludin was able to bind the ubiquitin ligase itch and degraded by proteasomes.34 Un-phosphorylated protein form are more liable for degradation. As hypoxia was thought to inhibit phosphorylation of both proteins, protein decrease could well be anticipated and subsequently proven in our Western blot. This procedure may pose another reason for decreased staining along cell boundaries.
We observed that pretreatment with Ang-1 antibodies could significantly block the effects of pericytes on occludin. Previous studies suggested that the attenuation of the Ang-1/Tie-2 pathway, a vital pathway in ECs and pericytes interaction, could lead to dysfunction of the TJs at the BBB in hypoxia. Two possible ways existed to modify this pathway. One is changing the secretion of Ang-1 from pericytes, and the other is inhibition of the interaction between Ang-1 and Tie-2. Regarding the latter, angiopoietin-2 is known to suppress angiopoietin-1 activity as an antagonist of Tie-2.35 Angiopoietin-2 produced by ECs increased prominently under hypoxia, and compared to this, the level of Ang-1 is sustained in PCM.36 Therefore, it is conceivable that angiopoietin-2 affects occludin expression under hypoxia by modifying Ang-1/Tie-2 pathway. This may explain the attenuation of Ang-1 effect under hypoxia in our experiment.
In conclusion, data obtained from this current study suggest that pericytes exerted its protective effects by upregulating occludin and ZO-1 levels as well as relocating both proteins. Ang-1 was highlighted as a potentially critical factor in accomplishing these effects on retinal ECs. As far as we know, this is the first report of pericytes' effects on retinal microvascular endothelia TJs properties. These findings provided important insights into the molecular mechanism of pericytes' paracrine effect under both normoxic and hypoxic conditions, and gave clues as to the selection of molecular therapy factors, such as Ang-1, to prevent TJs disruption.
References
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S . Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123: 1777–1788.
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S . Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141 (7): 1539–1550.
Barber AJ, ntonetti DA, Gardner TW . Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes: the Penn State Retina Research Group. Invest Ophthalmol Vis Sci 2000; 41: 3561–3568.
Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS et al. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 2004; 68 (3): 231–238.
Hawkins BT, Davis TP . The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57 (2): 173–185.
Janzer RC, Lobrinus JA, Darekar P, Juillerat L . Astrocytes secrete a factor inducing the expression of HT7-protein and neurothelin in endothelial cells of chorioallantoic vessels. Adv Exp Med Biol 1993; 331: 217–221.
Jeliazkova-Mecheva VV, Bobilya DJ . A porcine astrocyte/endothelial cell co-culture model of the blood–brain barrier. Brain Res Brain Res Protoc 2003; 12: 91–98.
D'Amore PA . Capillary growth: a two-cell system. Semin Cancer Biol 1992; 3 (2): 49–56.
Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest 2002; 110: 1619–1628.
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001; 153 (3): 543–553.
Sims DE . Diversity within pericytes. Clin Exp Pharmacol Physiol 2000; 27 (10): 842–846.
Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S et al. Angiopoietin-1 inhibits ocular neovascularization and breakdown of the blood–retinal barrier. Gene Therapy 2004; 11: 865–873.
Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 2000; 87 (7): 603–607.
Fischer S, Wobben M, Marti HH, Renz D, Schaper W . Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res 2002; 63: 70–80.
Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB . increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 2001; 42 (3): 853–859.
Frye CA, Patrick Jr CW . Isolation and culture of rat microvascular endothelial cells. In vitro Cell Dev Biol Anim 2002; 38 (4): 208–212.
Capetandes A, Gerritsen ME . Simplified methods for consistent and selective culture of bovine retinal endothelial cells and pericytes. Invest Ophthalmol Vis Sci 1990; 31 (9): 1738–1744.
Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA et al. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 2002; 283 (5): L1133–1142.
Brankin B, Campbell M, Canning P, Gardiner TA, Stitt AW . Endostatin modulates VEGF-mediated barrier dysfunction in the retinal microvascular endothelium. Exp Eye Res 2005; 81 (1): 22–31.
Grima J, Cheng CY . Testin induction: the role of cyclic 3′, 5′-adenosine monophosphate/protein kinase A signaling in the regulation of basal and lonidamine-induced testin expression by rat sertoli cells. Biol Reprod 2000; 63 (6): 1648–1660.
Tretiach M, van Driel D, Gillies MC . Transendothelial electrical resistance of bovine capillary endothelial cells is influenced by cell growth patterns. An ultrastructural study. Clin Exp Ophthalmol 2003; 31: 348–353.
Balabanov R, Dore-Duffy P . Role of the CNS microvascular pericyte in the blood–brain barrier. J Neurosci Res 1998; 53: 637–644.
Hirase T, Staddon JM, Saitou M, Anod-Akatsuka Y, Itoh M, Furuse M et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 1997; 110: 1603–1613.
McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S et al. Occludin is a functional component of the tight junction. J Cell Sci 1996; 109: 2287–2298.
Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W et al. Tight junctions are membrane microdomains. J Cell Sci 2000; 113: 1771–1781.
Jousen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 2002; 160 (5): 1683–1693.
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V et al. Isolation of angiopoietin1, a ligand for the Tie2 receptor by secretion trap expression cloning. Cell 1996; 87 (7): 1161–1169.
Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H et al. Recombinant angiopoietin1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest 2002; 110: 1619–1628.
Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res 2005; 1038 (2): 208–215.
Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N et al. Induction of blood–brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res 1999; 35: 155–164.
Fischer S, Wobben M, Kleinstuck J, Renz D, Schaper W . Effect of astroglial cells on hypoxia-induced permeability in PBMEC cells. Am J Physiol Cell Physiol 2000; 279: C935–C944.
Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW . Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999; 274: 23463–23467.
Clarke H, Soler AP, Mullin JM . Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci 2000; 113: 3187–3196.
Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fessler F et al. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin protein ligase itch. J Biol Chem 2002; 277: 10201–10208.
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60.
Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS . Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol 2000; 156: 2077–2089.
Acknowledgements
We thank Dan Chen and Xiaofeng Huang for their kindly help with the laser scanning confocal microscopy. Also, the authors would thank Dr Shaoyan Si and Dr Jing Wang from the department of biochemistry and molecular biology at the Fourth Military Medical University for their technical assistance. Last but not least, the authors would give much thanks to Dr Michael Roh of University of Alabama in Birmingham and Mr Lei Zhang for their hard work in English correction. This project was sponsored in part by an equipment donation from the Alexander von Humboldt Foundation, Germany (V-8151/02085).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Hui, Y., Guo, B. et al. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye 21, 1501–1510 (2007). https://doi.org/10.1038/sj.eye.6702716
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702716
Keywords
This article is cited by
-
Tissue oxygenation stabilizes neovessels and mitigates hemorrhages in human atherosclerosis-induced angiogenesis
Angiogenesis (2023)
-
Treatment with Atorvastatin During Vascular Remodeling Promotes Pericyte-Mediated Blood-Brain Barrier Maturation Following Ischemic Stroke
Translational Stroke Research (2021)
-
Pericytes in Brain Injury and Repair After Ischemic Stroke
Translational Stroke Research (2017)
-
Pericytes on the Tumor Vasculature: Jekyll or Hyde?
Cancer Microenvironment (2013)
-
Angiopoietin-1 protects myocardial endothelial cell function blunted by angiopoietin-2 and high glucose condition
Acta Pharmacologica Sinica (2011)