Abstract
Purpose
To compare the degree of posterior capsule opacification (PCO) and visual function between fellow eyes that received two different types of hydrophobic acrylic intraocular lenses (IOLs).
Methods
Forty-five patients underwent bilateral phacoemulsification and implantation of an Alcon AcrySof IOL (MA60AC) in one eye and an AMO Sensar IOL (AR40e) in the fellow eye. The PCO density value was measured using the Scheimpflug videophotography system at 1, 6, 12, and 24 months after surgery. The incidence of eyes that required a neodymium:YAG (Nd:YAG) laser capsulotomy, visual acuity, and contrast sensitivity with and without a glare source were also examined.
Results
The mean PCO value did not show a significant increase in either the AcrySof MA60AC or Sensar AR40e IOL groups, and was similar between the two groups throughout the 24-month follow-up period. The incidence of Nd:YAG laser capsulotomy was also the same between the groups. No significant difference was found in mean visual acuity between the two IOL groups during the follow-up, and there was no significant difference in photopic or mesopic contrast visual acuity with and without a glare source at 1 and 24 months after surgery.
Conclusion
The degree of PCO in eyes with an AcrySof IOL are almost the same as that in eyes with a Sensar IOL, with the results that visual acuity and contrast sensitivity with and without glare are similar.
Similar content being viewed by others
Introduction
It is well known that a hydrophobic acrylic intraocular lens (IOL) with a sharp optic edge (AcrySof; Alcon Surgical, Fort Worth, TX, USA) causes less posterior capsule opacification (PCO) than do the other IOLs.1, 2, 3, 4 Indeed, our previous studies verified that the degree of PCO as well as incidence of neodymium:YAG (Nd:YAG) laser posterior capsulotomy in eyes with the AcrySof IOL is significantly less than that in eyes with a PMMA, silicone, or hydrogel IOL.5, 6, 7
Based on these results, most surgeons now prefer to implant the hydrophobic acrylic IOL.8 Concomitant with this increase in preference, many kinds of hydrophobic acrylic IOL with a sharp optic edge design have been developed. However, the acrylic materials and optic edge design are different even between these newer hydrophobic acrylic IOLs. Of these hydrophobic acrylic lenses, the Sensar AR40e IOL (Advanced Medical Optics, Santa Ana, CA, USA) has a sophisticatedly modified optic edge design that may result in less pronounced photic phenomena and reduced PCO.9, 10 Our previous study did, in fact, demonstrate that PCO in eyes that received the AR40e IOL was very slight, which led to the maintenance of excellent visual function.11 Thus, the AcrySof and Sensar IOLs were now considered to be two of the most preferred IOLs with regard to PCO.
The purpose of the study described herein was to compare the degree of PCO and the Nd:YAG capsulotomy rate between eyes that received an AcrySof IOL with those implanted with the Sensar IOL. Furthermore, to compare the actual visual impairment owing to PCO, visual acuity and contrast sensitivity with and without a glare source were examined.
Patients and methods
Patients
All patients who were admitted sequentially to the Hayashi Eye Hospital for bilateral cataract surgery between March and July 2003 were screened for enrolment by a clinical research coordinator. Exclusion criteria were ocular pathology other than cataract, history of previous ocular surgery or inflammation, eyes scheduled for extracapsular cataract extraction, a pupil diameter less than 6.0 mm after mydriasis, patients with diabetes mellitus, and patients who could not be available for follow-up. Screening was continued until 50 patients who were to have bilateral phacoemulsification surgery and IOL implantation were recruited. The hospital's Institutional Review Board approved the study protocol, and all patients provided informed consent.
Randomization
All enrolled patients were randomly assigned the day before surgery to one of two groups. One group received an AcrySof IOL (MA60AC) in the left eye, and a Sensar IOL (AR40e) in the right eye. The other group received the MA60AC lens in the right eye and the AR40e lens in the left eye. Both of these IOLs are three-piece acrylic with a 6.0 mm round optic and PMMA-modified C-loops.
A clinical research coordinator generated a randomization code with equal numbers using random number tables, and, to ensure allocation concealment, kept concealed the assignment schedule until all data were collected. Patients and examiners were masked to randomization. The surgeon, who was also the data analyst, was masked to randomization before implantation. Operating room staff who allocated the IOL to the patients were not informed of the purpose of this study.
Surgical procedures
All surgeries were performed by a single surgeon (KH) using the same surgical procedure that has been described previously.12 First, a 3.5 mm straight scleral incision was made for IOL implantation. After incision, a continuous curvilinear capsulorrhexis measuring approximately 5.0 mm in diameter was accomplished using a bent needle. After hydrodissection, endocapsular phacoemulsification of the nucleus and aspiration of the residual cortex were performed. The wound was enlarged to 3.5 or 4.1 mm with a steel keratome for IOL implantation. The lens capsule was inflated with sodium hyaluronate 1% (Healon; Advanced Medical Optics), after which the IOL was placed into the capsular bag with an injector or folding forceps. After IOL insertion, the viscoelastic material was thoroughly evacuated. In this series, all surgeries were uneventful and all IOLs were implanted in the capsular bag.
Main outcome measures
The PCO density value in these patients was determined by a method described previously,6, 7 using the Scheimpflug videophotography system (EAS-1000; NIDEK, Gamagori, Japan) at 1, 6, 12, and 24 months after surgery. In brief, the examiner first obtained Scheimpflug slit images of the IOL at the 0°, 45°, 90°, and 135° meridians after full mydriasis. The highest quality image was then transferred to an online image analysis computer. The average scattering light density of the central 3.00 × 0.25-mm area of the posterior capsule, and of the central 3.00 × 0.25-mm area of the anterior optic surface was determined using the axial densitometry function of the image analysis computer. The PCO density value was expressed in computer-compatible tape steps: scattering light density obtained by densitometry divided the range from 0 to 255 (256 steps). The PCO density value in one cross-sectional image was determined by subtracting the scattering light density of the anterior IOL surface area from that of the posterior capsule area. The PCO density values of the four meridians were then averaged, and this averaged figure was taken as the PCO value of the eye. In addition, the area of the anterior capsule opening was also measured using the EAS-1000 system at 1 week after surgery, again using a method described previously.13
The incidence of eyes that required an Nd:YAG laser posterior capsulotomy was also examined. An Nd:YAG capsulotomy was performed when an eye lost two or more decimal lines of visual acuity or when the patient complained of blurred vision. For those patients who underwent Nd:YAG capsulotomy, the PCO value and visual acuity just before Nd:YAG capsulotomy were used for further statistical analysis. Best-corrected visual acuity on decimal charts was recorded at each visit, and the visual acuity was converted to a logarithm of minimal angle of resolution (logMAR) scale for statistical analysis. Contrast visual acuity and that measured in the presence of a glare source (glare visual acuity) were examined at 1 and 24 months after surgery using the Contrast Sensitivity Accurate Tester (CAT-2000; Menicon, Tokyo, Japan).10, 11 This device measures logMAR visual acuity using five contrast visual targets (100, 25, 10, 5, and 2.5%) under photopic and mesopic conditions. Measurement under photopic conditions was performed with chart lighting of 100 cd/mm2 and that under mesopic conditions with chart lighting of 5 cd/mm2. A glare light source of 200 lux was located in the periphery at 20° along the visual axis. The keratometric cylinder and objective refractive status were examined using an autokeratorefractometer (KR-7100; Topcon, Tokyo, Japan) and the pupil diameter was measured using a Colvard pupillometer (Oasis Medical, Glendora, CA, USA). All measurements were performed by ophthalmic technicians who were not aware of the purpose of this study and who were masked as to randomization.
Statistical analysis
The Mann–Whitney U test was used for comparison between the two IOL groups of the PCO value, logMAR visual acuity, contrast sensitivity, glare sensitivity, and other continuous variables. The Kruskal–Wallis test was used to compare differences in the PCO value and logMAR visual acuity at the various time points, and the Mann–Whitney U test was used to compare differences in the contrast visual acuity at 1 and 24 months after surgery. Discrete variables were compared using the χ2 test. Any differences showing a P-value of less than 0.05 were considered to be statistically significant.
Results
Of the 50 patients enrolled, one patient refused the examination, and four did not appear for examination because of a scheduling conflict. Therefore, 45 patients (90%) completed the 2-year follow-up and were included for the analysis.
The average age of the patients (±standard deviation (SD)) was 71.3±8.0 years, with a range of 53–84 years. There were 15 men and 30 women. No statistically significant differences were found between the two groups regarding the ratio of left to right eyes, keratometric cylinder, pupillary diameter, or the area of the capsulorrhexis opening (Table 1).
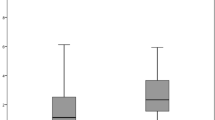
Figure 1 shows the mean (±SD) PCO value in the MA60AC and AR40e groups. The mean PCO value did not increase significantly in either group (P=0.0873 in the MA60AC group and P=0.3367 in the AR40e group), and when comparing the two groups, no significant difference was found in the mean PCO value throughout the 24-month follow-up period.
Mean (±SD) PCO value in the MA60AC and AR40e IOL groups. The mean PCO value did not increase significantly in either group (P=0.0873 in the MA60AC group; P=0.3367 in the AR40e group). When comparing groups, no significant difference was found in the mean PCO value throughout the 24-month follow-up period.
Of the 45 patients, only one (2.2%) required capsulotomy, and this individual underwent Nd:YAG capsulotomy in both eyes at 12 months after surgery. Therefore, the Nd:YAG capsulotomy rate of the two IOL groups was the same (P>0.9999 using the χ2 test).
Figure 2 shows the mean best-corrected visual acuity in the two IOL groups. Changes in mean visual acuity were not statistically significant in either group (P=0.9986 in the MA60AC group; P=0.9825 in the AR40e group). When comparing the MA60AC and AR40e groups, no significant difference was found in mean visual acuity throughout the follow-up period.
Mean (±SD) best-corrected visual acuity in the MA60AC and AR40e groups. Changes in mean visual acuity were not statistically significant in either group (P=0.9986 in the MA60AC group; P=0.9825 in the AR40e group). When comparing groups, no significant difference was found in mean visual acuity between the MA60AC and AR40e groups throughout the follow-up period.
Similar to our previously reported findings,10 in this series, we observed no significant difference between the two IOL groups in mean contrast visual acuity or glare visual acuity under either photopic or mesopic condition at 1 month after surgery. Furthermore, neither contrast visual acuity nor glare visual acuity worsened significantly from 1 to 24 months after surgery in either group, and at 24 months there was no significant difference between groups in the photopic or mesopic contrast visual acuity (Figure 3) or glare visual acuity (Figure 4).
Comparison of mean (±SD) contrast visual acuity between the MA60AC and AR40e groups under photopic (left) and mesopic (right) conditions at 24 months after surgery. No significant difference was found between the two IOL groups in mean contrast visual acuity under photopic (left) or mesopic (right) conditions at 24 months after surgery.
Comparison of mean (±SD) contrast visual acuity with a glare source between the MA60AC and AR40e groups under photopic (left) and mesopic (right) conditions at 24 months after surgery. No significant difference was found between the two IOL groups in mean contrast visual acuity with a glare source under photopic (left) and mesopic (right) conditions at 24 months after surgery.
Figure 5 illustrates retroillumination photographs showing bilateral eyes of a representative patient at 24 months after surgery. Both in an eye with the MA60AC IOL and in the fellow eye with the AR40e IOL, the posterior capsule is completely clear.
Discussion
Our study demonstrated that the degree of PCO in eyes that received an AcrySof MA60AC or Sensar AR40e IOL did not progress markedly during the 2-year follow-up. Consequently, the degree of PCO in eyes with an AcrySof MA60AC IOL and those with a Sensar AR40e IOL was virtually the same for up to 2 years after surgery. In addition, only one patient underwent Nd:YAG laser capsulotomy in both eyes. These results indicate that PCO in eyes with either AcrySof or Sensar IOL is very slight and is similar for up to at least 2 years after surgery.
Visual acuity was not impaired during the 2-year follow-up, and was similar in eyes with the AcrySof IOL and those with the Sensar IOL. Furthermore, contrast sensitivity and glare sensitivity under both photopic and mesopic conditions did not worsen significantly during follow-up, and were virtually the same between eyes with the AcrySof and Sensar IOLs at 2 years after surgery. These results indicate that visual function in eyes that received the AcrySof and Sensar IOLs did not deteriorate markedly owing to PCO for at least 2 years after surgery.
To verify clinical equivalence in PCO between the AcrySof and Sensar IOLs, we calculated the statistical power of our study to detect a clinically meaningful difference. When we suppose the PCO value of five CCT to be differences of clinical meaningful magnitude, the statistical power was calculated to be 99%. Furthermore, when we suppose the logMAR contrast visual acuity of 0.1 to be a clinically meaningful difference, the power was determined to be 87%. Thus, the powers were high enough to detect differences of clinical meaningful magnitude.
A number of studies have reported that the AcrySof IOL is associated with less PCO than the other IOLs.1, 2, 3, 4 Furthermore, most studies that compared the occurrence of PCO between IOLs, including ours,5, 6, 7 showed that it is least in those eyes with an AcrySof IOL.14, 15, 16, 17, 18, 19, 20, 21 However, until now there has been no study comparing the degree of PCO between fellow eyes with AcrySof and Sensar IOLs. In a recent study, we found that the degree of PCO is very slight in eyes with the Sensar IOL that has a sharp posterior edge.11 The present study further clarified that PCO, as well as visual function, did not worsen in eyes with either the AcrySof or the Sensar IOL for up to 2 years after surgery.
It has been shown clinically that the sharp edge of an IOL optic can prevent the invasion of PCO.11, 22, 23, 24, 25, 26 The AcrySof MA60AC has both sharp anterior and posterior edges, whereas the Sensar AR40e has a sharp posterior optic edge but a more round anterior optic edge. Because the degree of PCO was not different between eyes with these two different IOLs, the posterior optic edge must be more effective in preventing PCO than is the anterior optic edge. This can be explained by the fact that a sharp capsular bend is formed along the posterior optic edge, which prevents invasion of PCO, because both the anterior and posterior capsules adhere after surgery at the anterior side of the optic.11
Among the various hydrophobic acrylic IOLs, the chemical properties of the acrylic materials are different. For instance, it has been reported that the optic material of the AcrySof IOL tends to readily absorb intensely adhesive proteins, including fibronectin and vitronectin, which may lead to a strong adhesion of the optic to the lens capsule, and to subsequent prevention of PCO.27, 28, 29, 30 Surface whitening, which is known to often occur around the AcrySof optic,31 is supposed to consist of these proteins or water. On the other hand, the optic surface of the Sensar IOL seems to be smoother than that of the AcrySof IOL, because the Sensar IOL is made using cryo lathe cut and tumble polish methods, whereas the AcrySof IOL is made using cast moulding method. In addition, the acrylic polymer of the Sensar IOL is closer to polymethyl methacrylate than that of the AcrySof IOL. The differences in the optic materials, in conjunction with difference in optic edge design, result in the difference in the occurrence of PCO even among the hydrophobic acrylic IOLs. Indeed, we noted more PCO in eyes with a previous model of HOYA acrylic IOL (VA60CA and VA60CB; HOYA, Tokyo, Japan) than in those with an AcrySof IOL (unpublished data).
In conclusion, the degree of PCO in eyes that received either an AcrySof IOL or a Sensar IOLs is very slight, and is virtually the same between eyes with these IOLs. Furthermore, visual function does not worsen markedly because of PCO for at least 2 years after surgery in eyes with these IOLs. Based on these results, the AcrySof and Sensar IOLs are now two of the most preferred IOLs with regard to PCO. Accordingly, the use of these IOLs is recommended for patients at high risk of developing PCO, such as younger patients and those with diabetes. Further study is warranted to compare the degree of PCO in eyes implanted with an AcrySof or a Sensar IOL with that in eyes with other acrylic IOLs.
References
Oshika T, Suzuki Y, Kizaki H, Yaguchi S . Two year clinical study of a soft acrylic intraocular lens. J Cataract Refract Surg 1996; 22: 104–109.
Ursell PG, Spalton DJ, Pande MV, Hollick EJ, Barman S, Boyce J et al. Relationship between intraocular lens biomaterials and posterior capsule opacification. J Cataract Refract Surg 1998; 24: 352–360.
Hollick EJ, Spalton DJ, Ursell PG, Pande MV, Barman SA, Boyce JF et al. The effect of polymethylmethacrylate, silicone, and polyacrylic intraocular lenses on posterior capsular opacification 3 years after cataract surgery. Ophthalmology 1999; 106: 49–54; discussion by J Doe, 54–55.
Scmidbauer JM, Vargas LG, Apple DJ, Escobar-Gomez M, Izak A, Arthur SN et al. Evaluation of neodymium:yttrium–aluminum–garnet capsulotomies in eyes implanted with AcrySof intraocular lenses. Ophthalmology 2002; 109: 1421–1426.
Hayashi H, Hayashi K, Nakao F, Hayashi F . Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol 1998; 116: 1579–1582.
Hayashi K, Hayashi H, Nakao F, Hayashi F . Changes in posterior capsule opacification after poly(methyl methacrylate), silicone, and acrylic intraocular lens implantation. J Cataract Refract Surg 2001; 27: 817–824.
Hayashi K, Hayashi H . Posterior capsule opacification after implantation of a hydrogel intraocular lens. Br J Ophthalmol 2004; 88: 182–185.
Leaming DV . Practice styles and preferences of ASCRS members — 2003 survey. J Cataract Refract Surg 2004; 30: 892–900.
Franchini A, Gallarati BZ, Vaccari E . Computerized analysis of the effects of intraocular lens edge design on the quality of vision in pseudophakic patients. J Cataract Refract Surg 2003; 29: 342–347.
Hayashi K, Hayashi H . Effect of a modified optic edge design on visual function: textured-edge versus round-anterior slope-side edge. J Cataract Refract Surg 2004; 30: 1668–1674.
Hayashi K, Hayashi H . Posterior capsule opacification in the presence of an intraocular lens with a sharp versus rounded optic edge. Ophthalmology 2005; 112: 1550–1556.
Hayashi H, Hayashi K, Nakao F, Hayashi F . Elapsed time for capsular apposition to intraocular lens after cataract surgery. Ophthalmology 2002; 109: 1427–1431.
Hayashi K, Hayashi H . Intraocular lens factors that may affect anterior capsule contraction. Ophthalmology 2005; 112: 286–292.
Oner FH, Gunenc U, Ferliel ST . Posterior capsule opacification after phacoemulsification: foldable acrylic versus poly(methyl methacrylate) intraocular lenses. J Cataract Refract Surg 2000; 26: 722–726.
Kuçuksumer Y, Bayraktar S, Sahin S, Yilmaz OF . Posterior capsule opacification 3 years after implantation of an AcrySof and a MemoryLens in fellow eyes. J Cataract Refract Surg 2000; 26: 1176–1182.
Apple DJ, Peng Q, Visessook N, Werner L, Pandey SK, Escobar-Gomez M et al. Eradiation of posterior capsule opacification: documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology 2001; 108: 505–518.
Sundelin K, Friberg-Riad Y, Ostberg A, Sjostrand J . Posterior capsule opacification with AcrySof and poly(methyl methacrylate) intraocular lenses. Comparative study with a 3-year follow-up. J Cataract Refract Surg 2001; 27: 1586–1590.
Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A . Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg 2002; 28: 50–61.
Halpern MT, Covert D, Battista C, Weinstein AJ, Levinson RD, Yan L . Relationship of AcrySof acrylic and PhacoFlex silicone intraocular lenses to visual acuity and posterior capsule opacification. J Cataract Refract Surg 2002; 28: 662–669.
Ernest PH . Posterior capsule opacification and neodymium:YAG capsulotomy rates with AcrySof acrylic and PhacoFlex II silicone intraocular lenses. J Cataract Refract Surg 2003; 29: 1546–1550.
Zemaitiene R, Jasinskas V, Barzdziukas V, Auffarth GU . Prevention of posterior capsule opacification using different intraocular lenses (results of one-year clinical study). Medicina (Kaunas) 2004; 40: 721–730.
Kruger AJ, Schauersberger J, Abela C, Schild G, Amon M . Two year results: sharp versus rounded optic edges on silicone lenses. J Cataract Refract Surg 2000; 26: 566–570.
Buehl W, Findl O, Menapace R, Rainer G, Sacu S, Kiss B et al. Effect of an acrylic intraocular lens with a sharp posterior optic edge on posterior capsule opacification. J Cataract Refract Surg 2002; 28: 1105–1111.
Auffarth GU, Golescu A, Becker KA, Volcker HE . Quantification of posterior capsule opacification with round and sharp edge intraocular lenses. Ophthalmology 2003; 110: 772–780.
Buehl W, Menapace R, Sacu S, Kriechbaum K, Koeppl C, Wirtitsch M et al. Effect of a silicone intraocular lens with a sharp posterior optic edge on posterior capsule opacification. J Cataract Refract Surg 2004; 30: 1661–1667.
Sacu S, Menapace R, Findl O, Kiss B, Buehl W, Georgopoulos M . Long-term efficacy of adding a sharp posterior optic edge to a three-piece silicone intraocular lens on capsule opacification: five-year results of a randomized study. Am J Ophthalmol 2005; 139: 696–703.
Johnston RL, Spalton DJ, Hussain A, Marshall J . In vivo protein absorption to 2 intraocular lens materials. J Cataract Refract Surg 1999; 25: 1109–1115.
Linnola RJ, Sund M, Ylonen R, Pihlajaniemi T . Adhesion of soluble fibronectin, laminin, and collagen type IV to intraocular lens materials. J Cataract Refract Surg 1999; 25: 1486–1491.
Linnola RJ, Werner L, Pandy SK, Escobar-Gomez M, Znoiko SL, Apple DJ . Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. Part 1: histological sections. J Cataract Refract Surg 2000; 26: 1792–1806.
Linnola RJ, Werner L, Pandey SK, Escobar-Gomez M, Znoiko SL, Apple DJ . Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. Part 2: explanted intraocular lenses. J Cataract Refract Surg 2000; 26: 1807–1818.
Nishihara H, Yaguchi S, Onishi T, Chida M, Ayaki M . Surface scattering in implanted hydrophobic intraocular lenses. J Cataract Refract Surg 2003; 29: 1385–1388.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in any of the materials mentioned in this article and have received no financial support
Rights and permissions
About this article
Cite this article
Hayashi, K., Yoshida, M. & Hayashi, H. Comparison of posterior capsule opacification between fellow eyes with two types of acrylic intraocular lens. Eye 22, 35–41 (2008). https://doi.org/10.1038/sj.eye.6702496
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702496