Abstract
Purpose
To investigate the expression of the antiapoptotic and proapoptotic markers in diabetic retinas.
Methods
In total, 12 donor eyes from six subjects with diabetes mellitus, and 10 eyes from five nondiabetic subjects without known ocular disease serving as control subjects were examined. Immunohistochemical techniques were used with antibodies directed against cyclooxygenase-2 (Cox-2), Akt (protein kinase B), Mcl-1, Bad, cytochrome c, apoptosis-inducing factor (AIF), tumour necrosis factor receptor-1-associated death domain protein (TRADD), and Fas-associated death domain protein (FADD).
Results
In retinas from all subjects without diabetes, cytoplasmic immunoreactivity for the antiapoptotic molecules Cox-2, Akt, and Mcl-1 was noted in ganglion cells. Cytoplasmic immunostaining for Cox-2 was also noted in the retinal pigment epithelial cells. Weak immunoreactivity for the mitochondrial apoptogenic proteins cytochrome c, and AIF was noted in the inner segments of photoreceptors, in the inner one-third of the outer plexiform layer, in cells in the inner nuclear layer, in the inner plexiform layer, and in ganglion cells. There was no immunoreactivity for the other antibodies tested. All diabetic retinas showed de novocytoplasmic immunoreactivity for Bad in ganglion cells, and in occasional cells in the inner nuclear layer. Upregulation of cytochrome cand AIF immunoreactivity was noted. Cox-2, Akt, and Mcl-1 immunoreactivity was not altered in the diabetic retinas. There was no immunoreactivity for TRADD, and FADD.
Conclusions
Ganglion cells in diabetic and nondiabetic retinas express the antiapoptotic molecules Cox-2, Akt, and Mcl-1. Retinal ganglion cells express the proapoptotic molecule Bad in response to diabetes-induced neuronal injury. Diabetic retinas show upregulation of the mitochondrial proteins cytochrome c, and AIF.
Similar content being viewed by others
Main
Cell death by apoptosis, a tightly orchestrated event under the control of genetic programs, appears to play a prominent role in the development and pathogenesis of diabetic complications. Several in vitro and in vivo studies demonstrated that oxidative stress induced by hyperglycaemia leads to oxidative injury of neurons, which in turn activates the death pathways implicated in neuronal apoptosis.1, 2, 3 Retinas from diabetic rats showed increased oxidative stress, and administration of antioxidants inhibited the development of retinopathy.4, 5, 6 Increased apoptosis of neural retinal cells in experimental diabetes in rats and diabetes mellitus in humans was recently documented. This cell death by apoptosis gives rise to a chronic neurodegeneration in which neurons are lost in the diabetic retinas before other histopathology is detectable.7, 8
The molecular mechanisms that regulate neuronal survival and apoptosis in the retinas from human subjects with diabetes mellitus have not been completely identified. In a previous study, we demonstrated that ganglion cells in diabetic retinas expressed the apoptosis-promoting factors caspase-3, Fas, and Bax, suggesting that these cells are the most vulnerable population.9 A number of additional mediators that are either antiapoptotic or proapoptotic have been identified.10 They include among others the serine/threonine protein kinase Akt (also known as protein kinase B), cyclooxygenase-2 (Cox-2), Mcl-1, Bcl-2-associated death promoter (Bad), cytochrome c, apoptosis-inducing factor (AIF), tumour necrosis factor receptor-1-associated death domain protein (TRADD), and Fas-associated death domain protein (FADD).
Akt has been shown to protect neuronal cells against apoptosis by influencing the activity of several transcription factors implicated in regulating cell survival.11, 12, 13, 14, 15, 16 Several studies showed that Cox-2 functions as a survival factor by protecting cells from apoptosis.17, 18, 19, 20, 21 Overexpression of Mcl-1, a member of the Bcl-2 family,22 delays apoptosis by a broad array of agents.18, 21, 23, 24, 25, 26 Bad is a proapoptotic member of the Bcl-2 gene family that promotes apoptosis by binding to and inhibiting functions of antiapoptotic proteins Bcl-2 and Bcl-xL.10, 27 Mitochondria play a key role in the control of apoptotic cell death. Early during the apoptotic process, mitochondria can release several apoptogenic proteins, such as cytochrome c and AIF, into the cytosol.27, 28 The death domain containing adaptors TRADD and FADD act as pivotal proteins in the mechanism of ligand-induced programmed cell death (apoptosis) originating at the Fas (CD 95/APO-1) and tumour necrosis factor type 1 receptors. Recruitment of caspase-8 through TRADD and FADD results in caspase activation and subsequent apoptosis.29
Identifying the molecular mechanisms that regulate apoptosis of neural cells in diabetic retinas is beneficial for the development of therapeutic strategies that may provide a way to delay or prevent the onset of retinal neural cell death in patients with diabetes. Therefore, we used immunohistochemical techniques to study the expression of the apoptosis-related markers Akt, Cox-2, Mcl-1, Bad, cytochrome c, AIF, TRADD, and FADD in the retinas from diabetic patients and in the retinas from subjects without diabetes.
Methods
Study population
We obtained 12 human donor eyes postmortem from six subjects with diabetes mellitus. No subject had a history of retinal photocoagulation. We also obtained 10 eyes from five persons with no history of diabetes or of ocular disease, as determined by gross pathologic examination. Donor eyes were obtained and used in the study in accordance with the provisions of the Declaration of Helsinki for research involving the human tissue. Immediately after the specimens arrived in the laboratory, an incision was made 3 mm posterior to the limbus, and the cornea, iris, lens, and vitreous were gently removed. The retina and uveal tissue were dissected from the surrounding tissue, fixed in 4% paraformaldehyde, and embedded in paraffin.
Immunohistochemical staining
After deparaffinization, endogenous peroxidase was abolished with 2% hydrogen peroxide in methanol for 20 min, and nonspecific background staining was blocked by incubating the sections for 5 min in normal swine serum. For Cox-2 detection, the sections underwent heat-induced antigen retrieval with a microwave oven (three 5-min cycles in 10 mM Tris-EDTA buffer (pH 9) at 650 W). For Akt, Mcl-1, cytochrome c, AIF, TRADD, and FADD detection, antigen retrieval was performed by boiling the sections in 10 mM Tris-EDTA buffer (pH 9) for 30 min. Subsequently, the sections were incubated with the monoclonal and polyclonal antibodies listed in Table 1. The specificity of the antibodies used is indicated in Table 2. Optimum working concentration and incubation time for the antibodies were determined earlier in pilot experiments. For Cox-2, Akt, Mcl-1, cytochrome c, and Bad immunohistochemistry, the sections were incubated for 30 min with goat anti-rabbit or anti-mouse immunoglobulins conjugated to peroxidase-labelled dextran polymer (EnVision+; Dako, Carpinteria, CA, USA). For AIF, TRADD, and FADD immunohistochemistry, the sections were incubated for 30 min with the biotinylated secondary antibody and reacted with the avidin-biotinylated peroxidase complex (Dako). The reaction product was visualized by incubation for 10 min in 0.05 M acetate buffer at pH 4.9, containing 0.05% 3-amino-9-ethylcarbazole (Sigma-Aldrich, Bornem, Belgium) and 0.01% hydrogen peroxide, resulting in bright-red immunoreactive sites or 0.06% 3.3′-diaminobenzidine (Sigma) and 0.01% hydrogen peroxide resulting in brown immunoreactive sites. The slides were faintly counterstained with Harris haematoxylin. Finally, the sections were rinsed with distilled water and coverslipped with glycerol.
Omission or substitution of the primary antibody with an irrelevant antibody of the same species and staining with chromogen alone were used as negative controls. Sections from patients with colorectal carcinoma and breast cancer were used as positive controls. The sections from the control patients were obtained from patients treated at the University Hospital (University of Leuven, Belgium), in full compliance with the tenets of the Declaration of Helsinki.
All sections were examined by two independent observers (AMA, KG). One of them (KG) was unaware of the origin of the specimens. In case of disagreement, the results obtained by the blinded observer were used. Disagreement between the two observers was less than 5%. The staining was graded on the basis of the presence or absence of immunoreactivity, intensity of immunoreactivity, thickness of the staining, and the homogeneous or heterogenous character of staining.
Results
Donor's age and sex, type of diabetes, duration of diabetes, presence or absence of arterial hypertension, retinopathy status, cause of death, and death-to-enucleation times are summarized in Table 3. Nonproliferative diabetic retinopathy was documented to be present in two cases (Cases 1 and 2). The condition of the retina was unknown in three cases (Cases 3, 4 and 5). In these eyes, there were no histopathological changes suggestive of diabetic retinopathy. One case (Case 6) had no retinopathy.
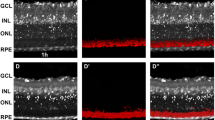
There was no staining in the negative control slides (Figure 1) and when the chromogen alone was applied. A summary of the results is given in Table 4. Similar findings were noted in retinas from all subjects without diabetes. Retinas from subjects without diabetes showed granular cytoplasmic immunoreactivity for Cox-2 in ganglion cells (Figure 2a) and in a few cells in the inner nuclear layer. Cytoplasmic immunostaining for Cox-2 was also noted in the retinal pigment epithelial cells and in the pigmented and nonpigmented layers of the ciliary body epithelium (data not shown). Granular cytoplasmic immunoreactivity for Akt, and Mcl-1 was noted in ganglion cells. Figure 2a shows a representative result of the expression of these mediators in ganglion cells. Occasional cells in the inner nuclear layer showed cytoplasmic immunoreactivity for Mcl-1. Weak granular immunoreactivity for cytochrome c, and AIF was noted in the inner segments of photoreceptors, in the inner one-third of the outer plexiform layer, in the cytoplasm of cells in the inner nuclear layer and ganglion cells, and in the inner plexiform layer. Figure 2b shows a representative result of the expression of these proteins. There was no immunoreactivity for Bad, TRADD, and FADD.
Photomicrograph of a retina from a diabetic subject. Negative control slide that was treated identically with an irrelevant antibody, showing no labelling. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments of photoreceptor cells; OS, outer segments of photoreceptor cells; RPE, retinal pigment epithelium. (Original magnification × 100).
Photomicrographs of a retina from a nondiabetic subject that was immunostained for cyclooxygenase-2, showing immunoreactivity in ganglion cells (arrows) (a), and apoptosis-inducing factor, showing immunoreactivity in inner segments of photoreceptors, in the inner one-third of outer plexiform layer, in cells in the inner nuclear layer, in the inner plexiform layer, and in ganglion cells (b). Abbreviations are defined in Figure 1 legend. (Original magnification × 100).
Similar findings were noted in all diabetic retinas. De novo cytoplasmic immuno-reactivity for Bad was observed in ganglion cells and in few cells in the inner nuclear layer (Figure 3a). Upregulation of granular cytochrome c, and AIF immunoreactivity was noted in the inner segments of photoreceptors, in the inner one-third of the outer plexiform layer, in cells in the inner nuclear layer, in the inner plexiform layer, and in ganglion cells. Figure 3b shows a representative result of the expression of these proteins. The distribution and intensity of immunoreactivity for Cox-2, Akt, and Mcl-1 was not altered in the diabetic retinas compared with retinas from subjects without diabetes. There was no immunoreactivity for TRADD and FADD.
Photomicrographs of a retina from a diabetic subject that was immunostained for Bad, showing immunoreactivity in ganglion cells (arrows) (a), and apoptosis-inducing factor, showing upregulation of apoptosis-inducing factor immunoreactivity (b). Abbreviations are defined in Figure 1 legend. (Original magnification × 100).
Discussion
In the present study, we demonstrated the following points: (1) retinal ganglion cells constitutively expressed the antiapoptotic molecules Akt, Cox-2, and Mcl-1; (2) diabetes induced a de novo expression of the proapoptotic molecule Bad in retinal ganglion cells; and (3) diabetic retinas showed upregulation of the mitochondrial proteins cytochrome c, and AIF.
The serine/threonine protein kinase Akt, a downstream effector of phosphatidylinositol (PI) 3-kinase (PI 3-kinase), appears to play a key role in mediating neuronal cell survival and neuroprotection.30 Orike et al11 demonstrated that the survival of adult sympathetic neurons in the absence of neurotrophic factors depends on PI 3-kinase/Akt signalling. Several studies showed that PI 3-kinase/Akt pathway plays a major role in mediating the survival response of retinal ganglion cells after axotomy to a variety of growth factors.12, 13, 14 In addition, apoptosis of retinal neurons induced by serum deprivation was reduced by insulin via activating the PI 3-kinase/Akt pathway.15 Furthermore, retinal PI 3-kinase/Akt signalling pathway was activated by optic nerve clamping and had a neuroprotective effect on injured retinal ganglion cells.16 In agreement with our results, immunoreactivity for phosphorylated Akt was detected in retinal ganglion cells in the injured retina.16
Cox enzymes mediate the production of prostaglandins from arachidonic acid. Two Cox isoforms Cox-1 and Cox-2 have been identified. Cox-1 is constitutively expressed in most tissues and is believed to be responsible for maintenance levels of prostaglandins for various housekeeping functions. In contrast, Cox-2 is the product of an immediate early gene that is rapidly inducible and tightly regulated. Cox-2 expression can be induced in various tissues by pathologic stimuli, such as bacterial lipopolysaccharide, proinflammatory cytokines, growth factors, hormones, and tumour promoters. Even though it is called the inducible isoform, Cox-2 is constitutively expressed in brain, spinal cord, testis, tracheal epithelia, and macula densa of kidney. In these tissues, Cox-2 is present without obvious stimulatory processes and is considered to be the dominating Cox isoform. Cox-2 has been shown to contribute constitutively to the physiologic regulation and development in these highly differentiated organ systems.17
Recent data showed that Cox-2 and the prostaglandins resulting from its enzymatic activation are involved in the control of cellular growth, angiogenesis, apoptosis, and development of neoplasia. Selective Cox-2 inhibitor attenuated the retinal angiogenesis that accompanies retinopathy of prematurity, and normal retinal development.31 These findings indicate that Cox-2 plays an important role in both developmental and pathologic retinal angiogenesis. In addition, several studies suggested a role of Cox-2 in neuronal function in the brain.32, 33 Furthermore, several studies have established a direct role for Cox-2 in rendering cells resistant to apoptosis by upregulating the expression of Mcl-1 through activation of the PI 3-kinase/Akt-dependent pathway.18, 21 In the present study, cytoplasmic immunoreactivity for Cox-2 was observed in retinal ganglion cells, in the retinal pigment epithelial cells, and in the pigmented and nonpigmented layers of the ciliary body epithelium in diabetic and nondiabetic retinas, confirming previous reports.31, 34, 35, 36 The expression of the antiapoptotic molecules Akt, Cox-2, and Mcl-1 in retinal ganglion cells may reflect the fact that neurons tend to be maintained for the entire life span of an individual.
In the present study, Bad was expressed in ganglion cells and in few cells in the inner nuclear layer in diabetic retinas. These observations suggest that retinal ganglion cells upregulate the expression of Bad in response to diabetes-induced neuronal injury. Similar observations were reported by Chen et al37 who demonstrated that neurotoxin-induced retinal neuronal degeneration induced a de novo expression of Bad in the retinal ganglion cells. In addition, transient retinal ischaemia by central retinal artery occlusion-induced upregulation of Bad expression in cells in the ganglion cell layer and inner nuclear layer.38
Mitochondria are key regulators in the process of cell death. Early during the apoptotic process, mitochondria can release a number of proapoptotic proteins from their intermembrane space, such as cytochrome c, and AIF.10, 27, 28 Cytoplasmic cytochrome c forms a complex, termed the apoptosome, with procaspase-9, and apoptotic protease activating factor-1 (Apaf-1), which activates caspase-3 and results in DNA fragmentation.10, 27 AIF has been shown to cause high molecular weight DNA fragmentation and chromatin condensation in cells and isolated nuclei in a caspase-independent manner.39, 40 The distribution of cytochrome c immunoreactivity observed in nondiabetic retinas is in agreement with previous human,41 and animal studies.42, 43 In addition, the distribution of AIF expression in nondiabetic retinas observed in the present study is consistent with a previous animal study.44 Diabetic retinas showed upregulation of cytochrome c and AIF immunoreactivity. Our data for cytochrome c in diabetic retinas are in agreement with several studies reporting increased cytochrome c immunoreactivity and activity in the retina postaxotomy and following optic nerve crush43, 45 suggesting that one of the early responses in the retina after optic nerve injury is to scale up the energy production. The study of Muranyi et al46 demonstrating that mitochondria dysfunction and mitochondria-initiated cell death pathway, which involves cytochrome c release, may play a key role in mediating diabetes-enhanced ischaemic brain damage is also in agreement with our findings. Several studies demonstrated that AIF is an important player in the regulation of caspase-independent neuronal cell death after cerebral hypoxia-ischaemia.47, 48 Furthermore, enforced expression of AIF can induce neuronal cell death in a caspase-independent manner, and blocking AIF function with neutralizing antibodies provides significant protection against cell death, suggesting that AIF may represent an important therapeutic target for neuroprotection after acute injury.47
Several in vitro and in vivo studies demonstrated that oxidative stress induced by hyperglycaemia is closely linked to apoptosis in a variety of cell types. Oxidative stress has been implicated in impaired mitochondrial function and activation of programmed cell death caspase pathway in diabetic neurons.1, 2, 3 There is overwhelming evidence for an involvement of reactive oxygen species in triggering mitochondria to release several essential players of apoptosis, such as cytochrome c, and AIF, into cytosol.28, 49 Several studies demonstrated that oxidative stress is increased in the retina with diabetes and is believed to play a significant role in the development of diabetic retinopathy.4, 5, 6 Administration of antioxidants inhibited the apoptosis-executer enzyme caspse-3 activation and inhibited the development of retinopathy.5, 6 Recently, it was demonstrated that diabetes-induced dysfunction of retinal mitochondria and increased the release of cytochrome c into the cytosolic fraction prepared from retina of rats.50
Although these observations, by their descriptive nature, do not allow a precise understanding of the function of these molecules, they do suggest that retinal ganglion cells possess protective mechanisms to guard against apoptosis, that retinal ganglion cells express Bad in response to diabetes-induced neuronal injury, and that diabetic retinas show upregulation of the mitochondrial proteins such as cytochrome c, and AIF.
References
Schmeichel AM, Schmelzer JD, Low PA . Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes 2003; 52: 165–171.
Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL . Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis 1999; 6: 347–363.
Vincent AM, Brownlee M, Russell JW . Oxidative stress and programmed cell death in diabetic neuropathy. Ann NY Acad Sci 2002; 959: 368–383.
Kowluru RA, Koppolu P, Chakrabarti S, Chen S . Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res 2003; 37: 1169–1180.
Du Y, Miller CM, Kern TS . Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 2003; 35: 1491–1499.
Kowluru RA, Koppolu P . Diabetes-induced activation of Caspase-3 in retina: effect of antioxidant therapy. Free Radic Res 2002; 36: 993–999.
Park S-H, Park J-W, Park S-J, Kim K-Y, Chung J-W, Chun M-H et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 2003; 46: 1260–1268.
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gradner TW et al. Neural apoptosis in the retina during experimental and human diabetes. J Clin Invest 1998; 102: 783–791.
Abu El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K . Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci 2004; 45: 2760–2766.
Schultz DR, Harrington Jr WJ . Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum 2003; 32: 345–369.
Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM . Role of PI3-Kinase, Akt and Bacl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J Cell Biol 2001; 154: 995–1005.
Kermer P, Klöcker N, Labes M, Bähr M . Insulin-like growth factor-1 protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 in vivo. J Neurosci 2000; 20: 722–728.
Nakazawa T, Tamai M, Mori N . Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3 K signaling pathways. Invest Ophthalmol Vis Sci 2002; 43: 3319–3326.
Weishaupt JH, Rodhe G, Polking E, Siren A-L, Ehrenreich H, Bähr M . Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 2004; 45: 1514–1522.
Barber AJ, Nakamura M, Wolpert EB, Reiter LEN, Seigel GM, Antonetti DA et al. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem 2001; 276: 32814–32821.
Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H et al. Intrinsic activation of PI3 K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res 2003; 26: 55–63.
Trifan OC, Hla T . Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med 2003; 7: 207–222.
Lin M-T, Lee R-C, Yang P-C, Ho F-M, Kuo M-L . Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1. J Biol Chem 2001; 276: 48997–49002.
Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA . Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res 2002; 62: 6323–6328.
Tang X, Sun YJ, Half E, Kuo MT, Sinicrope F . Cyclooxygenase-2 overexpression inhibits death receptor 5 expression and confers resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human colon cancer cells. Cancer Res 2002; 62: 4903–4908.
Nazeako UC, Guicciardi ME, Yoon J-H, Bronk SF, Gores GJ . Cox-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology 2002; 35: 552–559.
Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW . MCL 1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to Bcl 2. Proc Natl Acad Sci USA 1993; 90: 3516–3520.
Kuo ML, Chuang SE, Lin MT, Yang SY . The involvement of PI3-K/Akt-dependent up-regulation of Mcl-1 in the prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene 2001; 20: 677–685.
Chao JR, Wang JM, Lee SF, Peng HW, Lin YH, Chou CH et al. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol 1998; 18: 4883–4898.
Reynold JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A et al. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res 1994; 54: 6348–6352.
Zhou P, Qian L, Kozopas KM, Craing RW . Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 1997; 89: 630–643.
Van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P . Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun 2003; 304: 487–497.
Kannan K, Jain SK . Oxidative stress and apoptosis. Pathophysiology 2000; 7: 153–163.
Hsu H, Shu HB, Pan MG, Goeddel DV . TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF-receptor 1 signal transduction pathways. Cell 1996; 84: 299–308.
Franke TF, Kaplan DR, Cantley LC . P13K: downstream AKTion blocks apoptosis. Cell 1997; 88: 435–437.
Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE . Cox-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2003; 44: 974–979.
Chen C, Mayee JC, Bazan NG . Cyclooxinegase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol 2002; 87: 2851–2857.
Kaufmann WE, Worley PE, Pegg J, Bremer M, Isakson P . Cox-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proct Nat Acad Sci USA 1996; 93: 17–21.
Ju W-K, Neufeld AH . Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J Comp Neurol 2002; 452: 392–399.
Chin MS, Nagineni CN, Hooper LC, Detrick B, Hooks JJ . Cyclooxygenase-2 gene expression and regulation in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2001; 42: 2338–2346.
Maihöfner C, Schlötzer-Schrebardt U, Gühring H, Zeilhofer HU, Naumann GOH, Pahl A et al. Expression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyes. Invest Ophthalmol Vis Sci 2001; 42: 2616–2624.
Chen ST, Hsu JR, Hsu PC, Chuang JI . The retina is a novel in vivo model for studying the role of molecules of the Bcl-2 family in relation to MPTP neurotoxicity. Neurochem Res 2003; 28: 805–814.
Rickman DW, Nacke RF, Rickman CB . Characterization of the cell death promoter, Bad, in the developing rat retina and forebrain. Brain Res Dev Brain Res 1999; 115: 41–47.
Susin SA, Lorenzo HK, Zamzami N, Marzo J, Snow BE, Brothers GM et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397: 441–446.
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N et al. Mitochondria-nuclear translocation of AIF in apoptosis and necrosis. FASEB J 2000; 14: 729–739.
Andrews RM, Griffiths PG, Johnson MA, Turnbull DM . Histochemical localization of mitochondrial enzyme activity in human optic nerve and retina. Br J Ophthalmol 1999; 83: 231–235.
Isashiki Y, Nakagawa M, Higuchi I . Immunohistochemistry of the monkey retina with a monoclonal antibody against subunit V of cytochrome c oxidase. Acta Ophthalmol 1991; 69: 321–326.
Wang A-G, Lee C-M, Wang Y-C, Lin C-H, Fann M-J . Up-regulation of cytochrome oxidase in the retina following optic nerve injury. Exp Eye Res 2002; 74: 651–659.
Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y et al. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol 2001; 158: 1271–1278.
Cheung ZH, Yip HK, Wu W, So K-F . Axotomy induces cytochrome c release in retinal ganglion cells. Neuroreport 2003; 14: 279–282.
Muranyi M, Fujioka M, He QP, Han A, Yong G, Csiszar K et al. Diabetes activates cell death pathway after transient focal cerebral ischemia. Diabetes 2003; 52: 481–486.
Cregan SP, Fortin A, MacLaurin JG, Callghan SM, Cecconi F, Yu S-W et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol 2002; 158: 507–517.
Zhu C, Qiu L, Wang X, Hallin U, Candè C, Kroemer G et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem 2003; 86: 306–317.
Zhang C, Baffi J, Cousins SW, Csaky KG . Oxidant-induced cell death in retinal pigment epithelium cells mediated through the release of apoptosis-inducing factor. J Cell Sci 2003; 116: 1915–1923.
Kowluru R, Abbas N . Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci 2003; 44: 5327–5334.
Acknowledgements
This work was supported in part by the College of Medicine Research Centre, King Saud University. The authors thank Ms Christel van den Broeck for technical assistance, and Ms Connie B Unisa-Marfil for secretarial work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu El-Asrar, A., Dralands, L., Missotten, L. et al. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye 21, 238–245 (2007). https://doi.org/10.1038/sj.eye.6702225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702225
Keywords
This article is cited by
-
Mechanistic insights into the alterations and regulation of the AKT signaling pathway in diabetic retinopathy
Cell Death Discovery (2023)
-
Effect of retinol and α-tocopherol supplementation on photoreceptor and retinal ganglion cell apoptosis in diabetic rats model
International Journal of Retina and Vitreous (2022)
-
Molecular Mechanisms Mediating Diabetic Retinal Neurodegeneration: Potential Research Avenues and Therapeutic Targets
Journal of Molecular Neuroscience (2018)
-
Diabetes induces changes in neuroretina before retinal vessels: a spectral-domain optical coherence tomography study
International Journal of Retina and Vitreous (2015)
-
Apoptotic factors (Bcl-2 and Bax) and diabetic retinopathy in type 2 diabetes
Journal of Molecular Histology (2010)