Abstract
Purpose
Local treatment of uveal melanoma by radiotherapy involves the use of brachytherapy with radioactive plaques attached to the sclera, or proton irradiation. Both treatments induce growth arrest within the tumour and its slow involution over several years. Although ocular retention rates are excellent, regrowth of tumours due to resistance and neovascular glaucoma leads to enucleation of up to 10% of affected eyes. Proton irradiation involves part of the iris in most cases and we noticed that neovascularisation only occurred in the part of the iris that was not irradiated. We therefore conducted this study to determine the relationship between the development of iris neovascularisation and iris irradiation.
Methods
A total of 21 enucleation specimens from patients who had previously had proton irradiation were collected from the files of the Department of Pathology, Moorfields Eye Hospital during the 5-year period from 1994 to 1999. Sections of these eyes were assessed for VEGF-A, bFGF, and von Willebrand Factor (vWF) by immunohistochemistry. Ophthalmic notes and radiotherapy records were reviewed to assess the extent of iris irradiation.
Results
In all, 11 cases showed clinical evidence of iris neovascularisation and were selected for further study. Three of these eyes also showed clinical evidence of regrowth of the tumour. Histological evidence of iris neovascularization was noted in all 11 of the eyes examined, and was only present in the nonirradiated side of the iris in 8/11 eyes. NVI was present on both sides of the iris in three cases, but was less severe in the irradiated part. Expression of VEGF-A was at most weak within the tumour, but was present in the detached retina and in the epithelium of both ciliary body and iris. Some bFGF staining was noted around vessels in the iris stroma.
Conclusions
Our results suggest that irradiation leads to iris atrophy, and that atrophic, irradiated iris is resistant to the development of neovascularisation.
Similar content being viewed by others
Introduction
Treatment of primary uveal melanoma has improved significantly over the last 20 years and there are now several treatment options, of which enucleation is only one, with various types of radiotherapy (particularly plaque based) in use by most centres. However, this is unsuitable for very large tumours and for these eyes, proton beam irradiation has become a commonly preferred option in patients who wish to keep their eye.
The principle of proton beam irradiation is based on the improved dose distribution that can be achieved within the tumour, sparing intraocular structures due to maximum irradiation occurring at the apex of the Bragg peak.1, 2 The effect of proton beam therapy is growth arrest and the tumour only slowly involutes over many years.3 Histologically, the tumours show degenerative changes with balloon cells and lack of mitotic activity.3, 4, 5, 6, 7, 8 The histology of rubeosis iridis following proton therapy was first described postproton by Seddon et al,4 and also by Ferry et al,5 who noted iris neovascularisation (NVI) in both the treated and untreated area of the iris (70 GyE, given), although this case had iris involvement.
Although ocular retention rates over 90% have been reported, proton beam irradiation is not without its problems.9, 10, 11, 12 Regrowth of tumours due to resistance is relatively common, and many patients with larger tumours develop glaucoma secondary to NVI.10 This is usually unresponsive to treatment and necessitates enucleation of the eye. We treated a patient in whom NVI developed only in the nonirradiated part of the iris. The irradiated iris showed atrophic changes, but no evidence of irradiation. We have therefore gone back through our files to determine how prevalent this pattern is and to examine its pathogenesis.
Materials and methods
A total of 21 enucleation specimens from patients who previously had proton irradiation were collected from the files of the Department of Pathology, Moorfields Eye Hospital during the 5-year period from 1994 to 1999. All patients were treated at Clatterbridge using proton beam irradiation.13 All were cut either fixed (n=12) or prior to fixation (n=9) at 90° to the plane of the tumour, antero-posteriorly, and embedded as single block representing the middle portion of the eye including tumour, cornea, and optic disk. The paraffin-embedded tissue was cut at 6 μm to provide sections for diagnostic histology and immunohistochemistry.
Immunohistochemistry
Paraffin-processed sections (6 μm) of the eye were assessed for VEGF-A, Bfgf, and von Willebrand Factor (vWF) by immunohistochemistry using an avidin–biotin immunoalkaline phosphatase method as previously published.14 Briefly, sections were dewaxed in xylene, rehydrated through a series of alcohols, and washed in water. Sections were immunostained for VEGF-A using an affinity-purified rabbit IgG polyclonal antibody (SC152, Santa Cruz, Autogen Bioclear UK Ltd, Calne, Wilts, UK) at a 1 : 75 dilution. For this antibody, antigen retrieval was performed as described by Sheidow et al.15 Sections were placed in a microwave in citrate buffer pH 6.0 for 3.5 min on high power and 10 min on medium power, then 0.015% trypsin at 37°C for 45 min. A polyclonal antibody to Factor VIII related antigen (vWF, M616, Dako Ltd, High Wycombe, UK) was used at 1 : 500 following 30 min trypsinisation. Polyclonal anti-bFGF was obtained from Calbiochem/Oncogene Research Products (PC194L, Nottingham, UK) and used at a 1 : 20 dilution following trypsinisation of the sections for 15 min. Following repeated washing in Tris-buffered saline, (pH 7.6; TBS) for 10 min, nonspecific antibody binding was blocked by 0.1% bovine serum albumin (BSA) in TBS for 25 min. Primary incubations were performed for 1 h at room temperature in a humidified chamber. Slides were washed three times in TBS and the second antibody added. In all the cases, this was a biotinylated multilink antibody (Dako) used at a 1 : 300 dilution in TBS for 45 min. After washing, sections were incubated with a tertiary streptavidin-alkaline phosphatase reagent (Dako). The sections were again washed in TBS and incubated in Vector Red for 15 min according to the manufacturer's instructions (Vector Laboratories, Peterborough, England), washed and lightly counterstained with Mayer's haematoxylin for 5 s. Sections were then viewed by direct microscopy and the positivity of the iris and ciliary body was assessed qualitatively after ranking the specimens in the order of greatest staining.14 Immunohistochemical staining was graded as 0 (no staining), 1 (weak staining of >50% cells), 2 (intermediate staining of >50% cells), and 3 (strong staining of >50% of cells). Specificity of staining for VEGF-A was determined by competition with soluble recombinant cytokines (R&D Systems, each at up to 1 μg/ml) in three positive tumours with each antibody (data not shown).
Data analysis
The clinical notes made by the ophthalmologist were reviewed and the clinical data recorded in an ACCESS database, to which information from the histological and immunohistological examination was added. Radiotherapy plans were reviewed and the degree involvement of the iris in the radiation field noted. The results were crosstabulated to generate descriptive statistics.
Results
Clinical and radiotherapy data
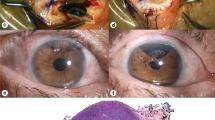
Of the 21 cases in our files over a 5-year period (1994–1999 inclusive), 11 showed clinical evidence of NVI and were selected for further studies. Partial iris atrophy was noted in 10 cases (Figure 1a). Three of these eyes also showed clinical evidence of regrowth of the tumour. The details of each of these cases are shown in Table 1. The median age of the patients was 60 years (range 26–73 years), and the group included five male and six female patients.
(a) Clinical appearance of the irradiated eye, showing atropy of one side of the iris with neovascularisation of the remainder. (b) Histology of irradiated iris, showing atrophy with no evidence of neovascularisation (vWF, × 100 original magnification). (c) Histology of nonirradiated iris showing neovascularisation (red) with peripheral anterior synechiae and ectropion uveae (vWF, × 100 original magnification).
Pathology
In all the cases, the tumours were large (median largest tumour diameter at enucleation=12 mm, range 7–25 mm) and either posteriorly placed (n=8) or peripheral, involving the ciliary body (n=3). Many were thin and appeared atrophic. This was reflected by the presence of mitotic activity, which was detected only in four tumours, three of which had clinical evidence of regrowth (Table 1). Involvement of the iris in the radiotherapy field ranged from 70 to 270° (median=180°).
As all eyes were cut antero-posteriorly through the tumour, one side of the iris was irradiated (always the same side as the tumour) in the sections examined. Histological evidence of NVI was noted in all the 11 eyes examined (Table 1), and was most severe on the nonirradiated side of the iris (the same side as the tumour) in all the cases. NVI was present on both sides of the iris in three cases.
Irradiated iris showed evidence of atrophy with reduced thickness and cellularity (Figure 1b). Atrophy was noted in 10 of the 11 eyes included. Rubeosis involved the atrophic iris in three of 11 cases, but was represented by the growth of capillary-like vessels over the anterior surface and did not involve the atrophic stroma (Figure 1c).
Immunohistochemistry
The presence of NVI histologically was confirmed in sections stained for vWF, which preferentially labels newly formed vessels. These sections (Figure 1) show small capillary-like vessels on the anterior surface of the iris, particularly at the pupillary margin. Expression of VEGF-A was at most weak within the tumour, but was present in the detached retina and in the epithelium of both ciliary body and iris. Some bFGF staining was noted around vessels in the iris stroma of eyes showing NVI.
Discussion
Our results suggest that irradiation leads to iris atrophy, and that atrophic, irradiated iris is resistant to the development of NVI. We noted bilateral growth of new vessels over atrophic iris in patients with severe NVI. This tended to be less intense in the nonirradiated area of the iris, and in these areas the degree of iris atrophy was less obvious. This is most likely to represent incomplete concordance between the plane of the histological section and the orientation of the radiotherapy field, rather than a biological phenomenon, although we cannot exclude the possibility that growth of blood vessels over atrophic iris may occur from nonirradiated NVI. Certainly, involvement of irradiated iris by tumour can be associated with NVI of the treated area,5 although this only explains one of our current cases with bilateral NVI.
It has long been suspected that release of angiogenic factors from the tumour or overlying retina might be responsible for NVI in uveal melanoma.16, 17, 18 We have previously noted high levels of soluble VEGF in the aqueous and vitreous of patients following proton beam irradiation, including one of the patients studied here in whom fresh vitreous was studied.19 We believe that this comes mainly from the detached retina, which stains strongly for VEGF-A.14 Foss et al10 identified retinal detachment and large tumour size as the factors most commonly associated with NVI following proton beam irradiation on the basis of multivariate analysis, confirming data from Coppin.16 This hypothesis is supported by our finding of higher levels of VEGF-A in vitreous than aqueous in these cases. bFGF is not found in vitreous in high amounts, but is likely to be produced locally as a secondary response to other cytokines, including VEGF.14, 19 Avoidance of iris irradiation can be achieved in some patients, particularly using narrow beam systems,20 but even then, NVI is a major cause of failure. We also note that NVI following proton beam irradiation can be successfully managed with pan-retinal photocoagulation.18, 21 Mild anterior uveitis has been noted after proton beam irradiation of larger tumours, and we cannot exclude the possibility that this could increase the exposure of the iris to proangiogenic cytokines, although this inflammation usually resolves rapidly.22
Cell proliferation is essential to angiogenesis, and irradiated tissues show evidence of reduced ability of their constituent cells to proliferate. This is as true of fibroblasts as it is of endothelial cells, both of which are essential to the wound-healing response.23 In proton-irradiated tissue, various changes have been observed previously. Endothelial cell loss has been noted in optic nerve following irradiation,24 and irradiation reduces mitotic and S-phase fraction in the tumour.3, 25
Current clinical practice is to avoid irradiation of the iris and anterior chamber as much as possible during proton therapy for melanoma.10 This is primarily based on the results reported by Char et al9 in several publications on large series of cases treated by proton therapy. However, this may represent a statistical bias due to the fact that eyes with larger tumours need a larger field of irradiation, and these are more likely to develop NVI, giving a misleading correlation between irradiation of the anterior chamber and the development of NVI (DH Char, personal communication). Iris irradiation has been associated with NVI or glaucoma due to pigment dispersion from atrophic iris in a number of studies.26, 27, 28 The former has generally been associated with a massive insult, such as extensive intraocular tumour invasion, often with iris involvement, or intravitreal haemorrhage. The pigment dispersion syndrome may occur due to radiation-induced cytolysis of the pigment epithelium, and may reflect direct toxicity,26 which is unlikely to occur with the dose distribution associated with modern proton beam irradiation.
Our results may be taken to suggest that irradiation of the whole iris in patients with a high risk of development of NVI and glaucoma would be preferable. We are not sure that this is true: damage to the angle or underlying ciliary body from radiation around 360° could produce more problems than it solves, although experimental work on this possibility should certainly be considered and in some patients it may be a reasonable therapy if they are warned that enucleation may be required at a later stage.
References
Gragoudas ES . Proton beam therapy of uveal melanomas. Arch Ophthalmol 1986; 104: 349–351.
Suit HD . Protons to replace photons in external beam radiation therapy? Clin Oncol (R Coll Radiol) 2003; 15: S29–S31.
Chiquet C, Grange JD, Ayzac L, Chauvel P, Patricot LM, Devouassoux-Shisheboran M . Effects of proton beam irradiation on uveal melanomas: a comparative study of Ki-67 expression in irradiated versus non-irradiated melanomas. Br J Ophthalmol 2000; 84: 98–102.
Seddon JM, Gragoudas ES, Albert DM . Ciliary body and choroidal melanomas treated by proton beam irradiation. Histopathologic study of eyes. Arch Ophthalmol 1983; 101: 1402–1408.
Ferry AP, Blair CJ, Gragoudas ES, Volk SC . Pathologic examination of ciliary body melanoma treated with proton beam irradiation. Arch Ophthalmol 1985; 103: 1849–1853.
Kincaid MC, Folberg R, Torczynski E, Zakov ZN, Shore JW, Liu SJ et al. Complications after proton beam therapy for uveal malignant melanoma. A clinical and histopathologic study of five cases. Ophthalmology 1988; 95: 982–991.
Liszauer AD, Brownstein S, Corriveau C, Deschenes J . A clinicopathological study of seven globes enucleated after primary radiation therapy for malignant melanoma of the choroid or ciliary body. Can J Ophthalmol 1990; 25: 340–344.
Saornil MA, Egan KM, Gragoudas ES, Seddon JM, Walsh SM, Albert DM . Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol 1992; 110: 1112–1118.
Char DH, Quivey JM, Castro JR, Kroll S, Phillips T . Helium ions versus iodine 125 brachytherapy in the managment of uveal melanoma. Ophthalmology 1993; 100: 1547–1554.
Foss AJ, Whelehan I, Hungerford JL, Anderson DF, Errington RD, Kacperek A et al. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br J Ophthalmol 1997; 81: 748–754.
Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM . Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol 2002; 120: 1665–1671.
Egger E, Zografos L, Schalenbourg A, Beati D, Bohringer T, Chamot L et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 2003; 55: 867–880.
Bonnett DE, Kacperek A, Sheen MA, Goodall R, Saxton TE . The 62 MeV proton beam for the treatment of ocular melanoma at Clatterbridge. Br J Radiol 1993; 66: 907–914.
Boyd SR, Tan DS, de Souza L, Neale MH, Myatt NE, Alexander RA et al. Uveal melanomas express vascular endothelial growth factor and basic fibroblast growth factor and support endothelial cell growth. Br J Ophthalmol 2002; 86: 440–447.
Sheidow TG, Hooper PL, Crukley C, Young J, Heathcote JG . Expression of vascular endothelial growth factor in uveal melanoma and its correlation with metastasis. Br J Ophthalmol 2000; 84: 750–756.
Coppin JM . Malignant melanoma and rubeosis iridis. Br J Ophthalmol 1973; 57: 815–824.
Gimbrone MA, Leapman SB, Cotran RS, Folkman J . Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumours. J Natl Cancer Inst 1973; 50: 219–228.
Gragoudas ES, Seddon J, Egan K, Glynn R, Munzenrider J, Austin-Seymour M et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology 1987; 94: 349–353.
Boyd SR, Tan D, Bunce C, Gittos A, Neale MH, Hungerford JL et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol 2002a; 86: 448–452.
Brovkina AF, Zarubei GD . Ciliochoroidal melanomas treated with narrow medical proton beam. Arch Ophthalmol 1986; 104: 402–404.
Kim MK, Char DH, Castro JL, Saunders WM, Chen GTY, Stone RD . Neovascular glaucoma after helium ion irradiation for uveal melanoma. Ophthalmology 1986; 93: 189–192.
Lumbroso L, Desjardins L, Levy C, Plancher C, Frau E, D'Hermies F et al. Intraocular inflammation after proton beam irradiation for uveal melanoma. Br J Ophthalmol 2001; 85: 1305–1308.
Constable PH, Crowston JG, Occleston NL, Cordeiro MF, Khaw PT . Long term growth arrest of human Tenon's fibroblasts following single applications of beta radiation. Br J Ophthalmol 1998; 82: 448–452.
Levin LA, Gragoudas ES, Lessell S . Endothelial cell loss in irradiated optic nerves. Ophthalmology 2000; 107: 370–374.
Pe'er J, Stefani FH, Seregard S, Kivela T, Lommatzsch P, Prause JU et al. Cell proliferation activity in posterior uveal melanoma after Ru-106 brachytherapy: an EORTC ocular oncology group study. Br J Ophthalmol 2001; 85: 1208–1212.
Bothman L . Glaucoma following irradiation. Arch Ophthalmol 1940; 23: 1198–1212.
Gragoudas ES, Caroll JM . Multiple choroidal metastasis from bronchial carcinoid treated with photocoagulation and proton beam irradiation. Am J Ophthalmol 1979; 87: 299–304.
Gragoudas ES, Seddon J, Goitein M, Verhey L, Munzenrider J, Urie M et al. Current results of proton beam irradiation of uveal melanomas. Ophthalmology 1985; 92: 284–291.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyd, S., Gittos, A., Richter, M. et al. Proton beam therapy and iris neovascularisation in uveal melanoma. Eye 20, 832–836 (2006). https://doi.org/10.1038/sj.eye.6702072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702072
Keywords
This article is cited by
-
Intravitreal bevacizumab for neovascular glaucoma in uveal melanoma treated by proton beam therapy
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
-
The effects of radiation on angiogenesis
Vascular Cell (2013)