Abstract
Purpose Nitric oxide (NO) mediates vascular endothelial growth factor (VEGF)-induced angiogenesis and vascular hyperpermeability. This study was undertaken to study the cellular distribution of inducible nitric oxide synthase (iNOS) and VEGF in the retinas from human subjects with diabetes mellitus. In addition, glial reactivity and peroxynitrite generation were detected by immunolocalization of glial fibrillary acidic protein (GFAP) and nitrotyrosine, respectively.
Methods Eight post-mortem eyes from four consecutive subjects with diabetes mellitus and eight eyes from four subjects without diabetes and without known ocular disease were prospectively collected and examined. We used immunohistochemical techniques and antibodies directed against iNOS, VEGF, GFAP, and nitrotyrosine.
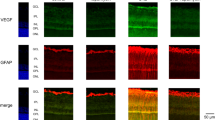
Results In retinas from all subjects without diabetes, weak GFAP immunoreactivity was confined to nerve fibre and ganglion cell layers. There was no immunoreactivity for iNOS, nitrotyrosine, and VEGF. All diabetic retinas showed GFAP induction in Müller cells and GFAP upregulation in nerve fibre and ganglion cell layers. All diabetic retinas showed cytoplasmic immunoreactivity for iNOS, and VEGF in ganglion cells, cells in the inner nuclear layer, and glial cells. In serial sections, ganglion cells and cells in the inner nuclear layer expressing VEGF were localized in the same area of iNOS-expressing ganglion cells and cells in the inner nuclear layer. Six retinas from three subjects with diabetes showed immunoreactivity for nitrotyrosine in vascular endothelial cells in inner retinal layer.
Conclusions iNOS and VEGF are colocalized in diabetic retinas. Increased GFAP immunoreactivity is a pathological event in the retina during diabetes.
Similar content being viewed by others
Introduction
Nitric oxide (NO) is a free radical, produced from L-arginine and molecular oxygen by NO synthases (NOS). Two isoforms of NOS are constitutively expressed in neurons and endothelial cells and are termed nNOS (NOS 1) and eNOS (NOS 3), respectively. These isoforms are calcium-dependent and produce low levels of NO for short periods in response to physiological stimuli. A third isoform of NOS, called inducible NOS (iNOS or NOS 2) is calcium-independent and is not constitutively expressed but requires cytokines or endotoxins for induction of its expression. Once expressed, iNOS produces large amounts of NO for prolonged periods of time that exerts cytotoxic and cytostatic effects and has been implicated in diverse functions associated with inflammation and injury.1,2 The high output of NO favours the reaction between NO and the superoxide anion to form peroxynitrite. The latter is a strong oxidant that can nitrate phenolic rings of tyrosine residues of proteins, leading to the formation of nitrotyrosine, which is easily detected with nitrotyrosine antibodies.3
The mechanisms by which diabetes induces retinopathy and blindness are not yet clear. However, some evidences suggest that an increased production of NO is involved in that process. In fact, we have observed substantial iNOS immunoreactivity in the retinas from human subjects with diabetes and nonproliferative diabetic retinopathy.4 In addition, increased expression of iNOS immunoreactivity and NO production in retinas from diabetic rats were reported.5,6 Several studies provided evidence implicating NO in blood–brain barrier breakdown7,8 and blood–retinal barrier breakdown.5 Moreover, NO has been implicated as a mediator of angiogenesis. In vitro and in vivo studies have shown that NO stimulates endothelial cell migration, proliferation, and differentiation into capillaries.9,10 One possible mechanism might involve the upregulation of αVβ3 integrin on endothelial cells, a critical mediator of cell-matrix adhesion and migration.10
Vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) is an endothelial cell-specific mitogen11 and strongly induces both physiological and pathological angiogenesis.12 VEGF is implicated strongly in the development of retinal and iris neovascularization in proliferative diabetic retinopathy. Eyes with nonproliferative and proliferative diabetic retinopathy showed an upregulated expression of VEGF and its mRNA by the retina.13,14 Several studies demonstrated that NO plays a critical role in VEGF-induced vascular hyperpermeability15,16,17,18 and angiogenesis.19,20,21,22 Furthermore, VEGF has been shown to induce the expression of iNOS23 and stimulate production of NO.18,24,25
The retina contains two types of macroglial cells. The most abundant are the Müller cells, which project from the retinal ganglion cell layer to the photoreceptors, whereas the astrocytes, which originate in the optic nerve and migrate into the retina during development,26 reside as a single layer adjacent to the inner limiting membrane. Glial cells provide structural and metabolic support for retinal neurons and blood vessels, and the cells become reactive in certain injury states.27,28,29 Several studies suggested that glial reactivity and altered glial metabolism are early pathological events in the retina during diabetes.30,31,32,33 The most constant manifestation of reactivity is the increase in immunoreactivity for the intermediate filament protein glial fibrillary acidic protein (GFAP).30
As NO has been shown to act as a crucial signal in the response of VEGF and as VEGF stimulates production of NO, we hypothesized that VEGF and iNOS are coexpressed in the retinas of human subjects with diabetes. To test our hypothesis, we used immunohistochemical techniques to study the distribution of VEGF and iNOS in the retinas from diabetic patients. In addition, we examined the expression of nitrotyrosine, a measure of in situ peroxynitrite modification of proteins. Furthermore, the expression of GFAP was compared in the retinas from diabetic patients and nondiabetic individuals.
Methods
We obtained eight human post-mortem eyes from four consecutive subjects with diabetes mellitus. No subject had a history of retinal photocoagulation. Nonproliferative diabetic retinopathy was documented to be present in two cases (Cases 1 and 2). The status of the retina was unknown in two cases (Cases 3 and 4). We also obtained eight eyes from four persons with no history of diabetes or of ocular disease as determined by gross pathologic examination. Immediately after the specimens arrived in the laboratory, an incision was made 3 mm posterior to the limbus, and the cornea, iris, lens, and vitreous were gently removed. The retina and uveal tissue were dissected free of surrounding tissue and divided into two parts. One part was fixed in 4% paraformaldehyde and embedded in paraffin and the other part was immediately snap-frozen in optimum cutting temperature compound (Tissue-Tek; Miles Laboratories, Elkhart, IN, USA) and maintained at −80°C until use.
Immunohistochemical techniques were used. After deparaffinization, endogenous peroxidase was abolished with 2% hydrogen peroxide in methanol for 3 min and nonspecific background staining was blocked by incubating the sections for 7 min in normal swine serum. Subsequently, sections were stained using a three-step avidin–biotin complex. Three sections were stained per antibody for each eye. For iNOS detection, the sections underwent heat-induced antigen retrieval with a microwave oven (three cycles of 5 min at 650 W). Subsequently they were incubated overnight with rabbit polyclonal anti-iNOS antibody (Transduction Laboratories, Lexington, KY, USA) diluted 1 : 100. The slides for VEGF detection were incubated for 30 min with rabbit polyclonal anti-VEGF antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) diluted 1 : 100. The slides for GFAP detection were incubated for 30 min with rabbit polyclonal anti-GFAP antibody (DAKO, Glostrup, Denmark) diluted 1 : 300. For the expression of nitrotyrosine, frozen sections were cut, dried overnight at room temperature, fixed in acetone for 10 min and incubated for 30 min with mouse monoclonal anti-nitrotyrosine (Cayman, Ann Arbor, MI, USA) diluted 1 : 50.
Incubation of primary antibodies was followed by incubation with the biotinylated secondary antibody and reacted with the avidin-biotinylated peroxidase complex. Secondary and tertiary reagents were purchased from DAKO (Denmark). All incubations were carried out for 30 min at room temperature and followed by three washes in phosphate-buffered saline (PBS), pH 7.2. The reaction product was visualized by incubation for 10 min in 0.05 mol/1 acetate buffer at pH 4.9, containing 3-amino-9-ethyl-carbazole 0.05% (AEC, Sigma-Aldrich, Bornem, Belgium) and hydrogen peroxide 0.01%, resulting in bright-red immunoreactive sites. The slides were faintly counterstained with Mayer's haematoxylin. Finally, the sections were rinsed with distilled water and coverslipped with glycerol. Control slides were treated in an identical manner, except that an irrelevant antibody was used in the first step or the primary antibody was omitted. Evaluation of the retinas was performed by two independent observers (AMA and KG). One of them (KG) was unaware of the origin of the specimens.
Results
Donor age and sex, type of diabetes, duration of diabetes, cause of death, and death to enucleation time are summarized in Table 1. In retinas from all subjects without diabetes, weak immunoreactivity for GFAP was noted very close to internal limiting membrane in nerve fibre layer and ganglion cell layer. Müller cell processes were not stained (Figure 1). There was no immunoreactivity for iNOS, nitrotyrosine, and VEGF.
All diabetic retinas showed strong immunoreactivity for GFAP in nerve fibre layer and ganglion cell layer, which was more pronounced around blood vessels in the innermost retinal layer. Many of these vessels were dilated (Figure 2). Müller cell processes that extend radially throughout the retina showed moderate GFAP immunoreactivity (Figure 3).
In all diabetic retinas, cytoplasmic granular immunoreactivity for iNOS was detected in ganglion cells (Figure 4) and in cells in the inner nuclear layer. In addition, cytoplasmic iNOS immunoreactively was observed in GFAP-positive Müller cell processes (Figure 5) and in GFAP-positive cells around blood vessels in the innermost retinal layer in serial sections (Figure 6). Strong immunoreactivity for nitrotyrosine was localized to endothelial cells of blood vessels in the innermost retinal layer in six retinas from three subjects (Cases 2, 3, and 4) (Figure 7).
Granular cytoplasmic immunoreactivity for VEGF was observed in ganglion cells and in cells in inner nuclear layer. In addition, numerous cell processes within nerve fibre layer, inner plexiform layer, and inner nuclear layer showed granular cytoplasmic immunoreactivity for VEGF, which was similar to the pattern of cells that stained for GFAP in serial sections (Figure 8).
In serial sections, ganglion cells and cells in the inner nuclear layer expressing VEGF were localized in the same area of iNOS-expressing ganglion cells and cells in the inner nuclear layer. Control sections did not immunostain, whether we omitted the primary antibody or substituted an irrelevant antibody for the primary antibody.
Discussion
This study showed that, using the procedures described, iNOS, and VEGF have similar distributions and similar immunohistochemical staining patterns with cytoplasm-associated immunoreactivity in ganglion cells, cells in the inner nuclear layer, and glial cells in the retinas from human subjects with diabetes. It has been shown in animal studies that iNOS protein is localized at the ganglion layer, at the inner nuclear layer, and at the outer nuclear layer in the retinas of diabetic rats.5 iNOS immunoreactivity was observed in the retina in other ischaemic retinopathies as well. Retinal ischaemia induced by common carotid artery occlusion in rats induced iNOS expression in Müller cells and retinal ganglion cells.34 In a murine model of oxygen-induced retinopathy of prematurity, iNOS mRNA was expressed in ischaemic retina.35 In a previous study we have shown iNOS immunoreactivity in retinal Müller glial cells from human patients with diabetes and nonproliferative diabetic retinopathy using immunohistochemistry and heat-induced antigen retrieval with a microwave oven and 30 min incubation with the antibody.4 In the present study, we used additionally overnight incubation in order to increase antigen retrieval. This resulted in enhanced immunoreactivity without affecting the quality of staining.
In the present study, iNOS and VEGF immunoreactivities were colocalized in ganglion cells and in cells in the inner nuclear layer in serial sections. This colocalization can be explained in two ways, either through a common induction pathway or through a sequential relation in time between both molecules. Ischaemia has been shown to induce the expression of iNOS34,35 and VEGF36 in the ischaemic retina. Yet, other studies demonstrated that VEGF induced the expression of iNOS in human endothelial cells.23 Recently, several studies demonstrated expression of VEGF receptors in retinal ganglion cells, inner nuclear layer, and Müller cells.37,38
Induction of iNOS through VEGF stimulates production of NO from rabbit and human endothelial cells through activation of tyrosine kinases and an increase in intracellular calcium.18,24,25 Several studies demonstrated that NO plays a critical role in VEGF-induced vascular hyperpermeability and angiogenesis. In vitro studies showed that VEGF increases permeability17 and elevates hydraulic conductivity15 of cultured bovine retinal microvascular endothelial cells through a signalling mechanism that involves NO. In vivo studies demonstrated that an NOS inhibitor blocked VEGF-induced vascular hyperpermeability in all ocular and nonocular tissues.16 Since the expression of iNOS protein was mainly localized at the level of the retinal vessels, it is possible that the overproduction of NO by the iNOS isoform contributes to blood–retinal barrier breakdown in the retinas of human subjects with diabetes. The mitogenic action of VEGF on endothelial cells is NO mediated since NOS inhibitors block the VEGF-induced proliferation.19 The experiments of in vivo angiogenesis indicate that NO is downstream imperative of VEGF-induced angiogenesis. Systemic administration of NOS inhibitors to rabbits bearing a corneal implant blocked VEGF-induced angiogenesis.20 In a rat model of experimental peripheral arterial insufficiency, normal NO production was essential for the enhanced vascular remodelling induced by exogenous VEGF.39 In a murine model of operatively induced hindlimb ischaemia, impaired angiogenesis in an eNOS knockout mice was not improved by administration of VEGF.22
Recent evidence indicates that iNOS plays a crucial role in ocular neovascular disease by inducing retinal vaso-obliteration and enhancing pathological intravitreal neovascularization. In a murine model of retinopathy of prematurity, iNOS expression was found to inhibit angiogenesis locally in the avascular retina mediated by a downregulation of VEGF receptor 2, and to encourage intravitreal neovascularization. iNOS inhibitors enhanced angiogenesis in the ischaemic retina and inhibited pathological intravitreal neovascularizaton. In addition, pathological intravitreal neovascularization was significantly reduced in iNOS knockout mice.35 In addition, Brooks et al40 demonstrated that oxygen-induced retinal vaso-obliteration was significantly reduced by an NOS inhibitor, strongly supporting a putative role for NO in the retinal vaso-obliterative process. Nitrotyrosine is derived from peroxynitrite, which is formed by the interaction of NO and the superoxide anion radical. Peroxynitrite is implicated in the pathogenesis of neurotoxicity in neurodegenerative diseases41 and central nervous system inflammation.42 In a murine model of oxygen-induced retinopathy of prematurity, nitrotyrosine expression occurred in the inner retina early in the course of vaso-obliteration.40 In the present study, nitrotyrosine immunoreactivity was localized to the vascular endothelium in inner retinal layer consistent with a putative role in the pathogenesis of diabetic microangiopathy. The presence of nitrotyrosine suggests that overexpression of iNOS is associated with NO release in the retinas from human subjects with diabetes.
In the nondiabetic retinas, weak GFAP immunoreactivity was confined to the innermost retinal layers. Müller cell processes were not stained. Our observations are consistent with results obtained in human,43 primate,44 and rat30,31,32,33 retinas. Diabetic retinas showed GFAP upregulation in nerve fibre and ganglion cell layers, and GFAP induction in Müller cells indicating glial reactivity. Increased GFAP immunoreactivity has also been observed in retinas from diabetic subjects43 and rats with experimental diabetes.30,31,32,33 Several studies showed increased GFAP immunoreactivity in early experimental diabetic retinopathy suggesting that glial reactivity is one of the earliest pathological events in the retina during diabetes.30,31,32,33 The mechanism of the increased GFAP content in the retinas of diabetic subjects is as yet unclear. Rungger-Brändle et al31 proposed that leakage from the vascular bed not only leads to increased glucose levels within the neural parenchyma capable of inducing glial reactivity by itself but sets free a number of blood-derived factors that are potential additional triggers. As glial cells are implicated in the maintenance of the blood–retinal barrier,27 our observations of glial reactivity, and expression of iNOS and VEGF by glial cells open the interesting possibility that glial malfunction could contribute to the breakdown of the blood–retinal barrier in diabetic retinopathy. It has been suggested that dysfunction of retinal astrocytes is of importance in ischaemic retinal neovascularization.45
In conclusion, these findings indicate that iNOS and VEGF are remarkably colocalized in the retinas from human subjects with diabetes. Expression of iNOS with a consequent increase in NO production, together with VEGF, may contribute to the increased blood–retinal barrier permeability and retinal angiogenesis. In addition, activation of glial cells occurs in the retinas from subjects with diabetes and may be involved in the process of tissue damage.
References
Nathan C . Nitric oxide as a secretory product of mammalian cells. FASEB J 1992; 6: 3051–3064.
Nathan C . Inducible nitric oxide synthase: what difference does it make? J Clin Invest 1997; 100: 2417–2423.
Beckman JS, Koppenol WH . Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol 1996; 271: C1424–C1437.
Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K . Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol 2001; 132: 551–556.
Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC . Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood–retinal barrier permeability. Nitric Oxide 2000; 4: 590–596.
Kowluru RA, Engerman RL, Kern TS . Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr Eye Res 2000; 21: 814–819.
Nag S, Picard P, Stewart DJ . Expression of nitric oxide synthases and nitrotyrosine during blood–brain barrier breakdown and repair after cold injury. Lab Invest 2001; 81: 41–49.
Boje KMK . Inhibition of nitric oxide synthase attenuates blood–brain barrier disruption during experimental meningitis. Brain Res 1996; 720: 75–83.
Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 1994; 94: 2036–2044.
Lee PC, Kibbe MR, Schuchert MJ, Stolz DB, Watkins SC, Griffith BP et al. Nitric oxide induces angiogenesis and upregulates αVβ3 integrin expression on endothelial cells. Microvasc Res 2000; 60: 269–280.
Ferrara N, Houck L, Jakeman L, Leung DW . Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev 1992; 13: 18–32.
Dovark MF, Brown LF, Detmar M, Dovark AM . Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: 1029–1039.
Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E . Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 1996; 80: 241–245.
Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW . Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 1997; 38: 36–47.
Lakshminarayanan S, Antonetti DA, Gardner TW, Tarbell JM . Effect of VEGF on retinal microvascular endothelial hydraulic conductivity: the role of NO. Invest Ophthalmol Vis Sci 2000; 41: 4256–4261.
Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR . Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci 1999; 40: 689–696.
Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB . VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci 1999; 40: 157–167.
Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M et al. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol 1998; 153: 381–394.
Shizukuda Y, Tang S, Yokota R, Ware JA . Vascular endothelial growth factor-induced endothelial cell migration and proliferation depend on a nitric oxide-mediated decrease in protein kinase Cδ activity. Circ Res 1999; 85: 247–256.
Ziche M, Morbidelli L, Choudhuri R, Zhang H-T, Donnini S, Granger HJ et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997; 99: 2625–2634.
Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M . Nitric oxide mediates mitogenic effect of VEGF on coronary vascular endothelium. Am J Physiol 1996; 270: H411–H415.
Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C et al. Nitric oxide synthase modulates angiogenesis response to tissue ischemia. J Clin Invest 1998; 101: 2567–2578.
Kroll J, Waltenberger J . VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem Biophys Res Commun 1998; 252: 743–746.
Van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, LeKutat C et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 1997; 95: 1030–1037.
Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC . Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997; 100: 3131–3139.
Watanabe T, Raff MC . Retinal astrocytes are immigrants from the optic nerve. Nature 1988; 332: 834–837.
Tout S, Chan-Ling T, Hollander H, Stone J . The role of Müller cells in the formation of the blood–retinal barrier. Neuroscience 1993; 55: 291–301.
Newman E, Reichenbach A . The Müller cell: a functional element of the retina. Trends Neurosci 1996; 19: 307–312.
Kawasaki A, Otori Y, Barnstable CJ . Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci 2000; 41: 3444–3450.
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanaser D, Strother JM et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes 1998; 47: 815–820.
Runnger-Brändle E, Dosso AA, Leuenberger PM . Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 2000; 41: 1971–1980.
Barber AJ, Antonetti DA, Gardner TW, The Penn State Retina Research Group. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci 2000; 41: 3561–3568.
Zeng XX, Ng YK, Ling EA . Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci 2000; 17: 463–471.
Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T . Nitric oxide synthase expression in ischaemic rat retinas. Jpn J Ophthalmol 2000; 44: 235–244.
Sennlaub F, Courtois Y, Goureau O . Inducible nitric oxide synthase mediates the change from retinal to vitreal neovascularization in ischaemic retinopathy. J Clin Invest 2001; 107: 717–725.
Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994; 145: 574–584.
Ogata N, Yamanaka R, Yamamoto C, Miyashiro M, Kimoto T, Takahashi K et al. Expression of vascular endothelial growth factor and its receptor, KDR, following retinal-ischemia-reperfusion injury in the rat. Curr Eye Res 1998; 17: 1087–1096.
Still AW, Simpson DA, Boocock C, Gardiner TA, Murphy GM, Archer DB . Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours. J Pathol 1998; 186: 306–312.
Yang HT, Yan Z, Abraham JA, Terjung RL . VEGF121- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol Heart Circ Physiol 2001; 28: H1097–H1104.
Brooks SE, Gu X, Samuel S, Marcus DM, Bartoli M, Huang PL et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci 2001; 42: 222–228.
Torreilles F, Salman-Tabcheh S, Guerin M-C, Torreilles J . Neurodegenerative disorders: the role of peroxynitrite. Brain Res Rev 1999; 30: 153–163.
Van der Veen RC, Hinton DR, Incardonna F, Hofman FM . Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J Neuroimmunol 1997; 77: 1–7.
Mizutani M, Gerhardinger C, Lorenzi M . Müller cell changes in human diabetic retinopathy. Diabetes 1998; 47: 445–449.
Tanihara H, Hangai M, Sawaguchi S, Abe H, Kageyama M, Nakazawa F et al. Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Opthalmol 1997; 115: 752–756.
Stone J, Chan-Ling T, Itin A, Gnessin H, Keshet E . Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1996; 37: 290–299.
Acknowledgements
We thank Ms Christel Van den Broeck for technical assistance and Ms Connie B Unisa-Marfil for secretarial work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu El-Asrar, A., Meersschaert, A., Dralands, L. et al. Inducible nitric oxide synthase and vascular endothelial growth factor are colocalized in the retinas of human subjects with diabetes. Eye 18, 306–313 (2004). https://doi.org/10.1038/sj.eye.6700642
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700642
Keywords
This article is cited by
-
Nitric oxide and TNF-α are correlates of diabetic retinopathy independent of hs-CRP and HbA1c
Endocrine (2020)
-
Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice
Stem Cell Research & Therapy (2016)
-
Chronic hyperglycemia inhibits vasoregression in a transgenic model of retinal degeneration
Acta Diabetologica (2014)
-
Involvement of Müller glial cells in epiretinal membrane formation
Graefe's Archive for Clinical and Experimental Ophthalmology (2009)
-
Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes
Diabetologia (2007)