Abstract
Purpose To compare (a) the clinical effectiveness and (b) cost effectiveness of the two models in screening for diabetic retinopathy.
Methods (a) Retrospective analysis of referral diagnoses of each screening model in their first respective years of operation and an audit of screen positive patients and a sample of screen negatives referred to the hospital eye service from both screening programmes. (b) Cost effectiveness study.
Participants (1) A total of 1643 patients screened in the community and in digital photography clinics; (2) 109 consecutive patients referred to the Diabetic Eye Clinic through the two existing models of diabetic retinopathy screening; (3) 55 screen negative patients from the optometry model; (4) 68 screen negative patients audited from the digital photography model.
Results The compliance rate was 45% for optometry (O) vs50% for the digital imaging system (I). Background retinopathy was recorded at screening in 22% (O) vs17% (I) (P=0.03) and maculopathy in 3.8% (O) vs1.7% (I) (P=0.02). Hospital referral rates were 3.8% (O) vs4.2% (I) Sensitivity (75% for optometry, 80% for digital photography) and specificity (98% for optometry and digital photography) were similar in both models. The cost of screening each patient was £23.99 (O) vs£29.29 (I). The cost effectiveness was £832 (O) vs£853(I) in the first year.
Conclusion The imaging system was not always able to detect early retinopathy and maculopathy; it was equally specific in identifying sight-threatening disease. Cost effectiveness was poor in both models, in their first operational year largely as a result of poor compliance rates in the newly introduced screening programme. Cost effectiveness of the imaging model should further improve with falling costs of imaging systems. Until then, it is essential to continue any existing well-coordinated optometry model.
Similar content being viewed by others
Introduction
Diabetic retinopathy is the leading cause of blindness of working age patients in the UK.1 Laser photocoagulation is effective if retinopathy is detected before irreversible changes take place.2 Improving metabolic control in patients with mild retinopathy slows the progression of retinopathy,3 indicating a case for the detection of early background retinopathy. In a survey of ophthalmologists in England and Wales carried out in 1999, there was no programme of diabetic retinopathy screening in 23% and in only 64% were the results routinely sent to the general practitioner.4 In 1999, the UK National Screening Committee asked the British Diabetic Association (now Diabetes UK) to convene an advisory panel to produce a model for a cost-effective national screening programme. The panel's recommendations are now published on the national screening committee's website.5 Digital retinal photography is the preferred modality, and a national programme, to be rolled over a period of 3–4 years, has been proposed.
In the Warrington NHS Health Trust catchment area (total population 306 401, 1991 census), two models of screening for diabetic retinopathy have been operating in parallel for its two component areas—Warrington and Halton—for geographic and logistical reasons. For the Warrington area, an optometric screening programme run by accredited optometrists is in operation since November 1995, whereas a digital photographic screening was introduced in November 1998 for the Halton area. This is a unique setting among the health trusts in the UK and affords us an opportunity to compare the two methods in terms of effectiveness and cost effectiveness in their respective first years of running.
Methods
Screening programme
(a) Optometric screening. This was carried out by optometrists, using slit-lamp biomicroscopy, in the Warrington area who have been accredited for screening following attendance and subsequent assessment at the Retinal Eye Clinic in Warrington Hospital. The accreditation is based on the British Diabetic Association model.6 Patients are directly referred to the hospital diabetic eye service according to a set protocol.
Referable retinopathy (RR) is defined as: background retinopathy with macular involvement; background retinopathy without macular involvement if large circinate or plaque hard exudates within the major temporal vascular arcades; and background retinopathy and reduced visual acuity (<6/12 Snellen) not corrected by pin hole (suggestive of macular oedema).
Preproliferative retinopathy involves: five or more cotton wool spots and/or the presence of venous abnormalities (eg tortuosity or beading), or intraretinal microvascular abnormalities and/or extensive intraretinal haemorrhages.
Proliferative retinopathy involves: advanced diabetic eye disease (vitreous haemorrhage, fibrous tissue, recent retinal detachment and rubeosis iridis).
The above findings are regarded as sight-threatening diabetic eye disease—STDR.
Suspected glaucoma: intraocular pressure of more than 22 mmHg ± a cup : disc ratio of >0.6 or an asymmetry of >0.2.
Any other lesion that the observer cannot interpret with reasonable certainty: incidental retinal vascular problems/ naevus.
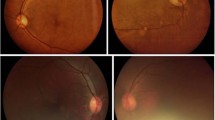
(b) Digital photography for Halton residents. This was carried out by the Halton Eye Screening Project (HESP) based at Halton Hospital using a fixed digital camera (Topcon nonmydriatic, model TRC-NW5S, Sony video head 3CCD DXC-950P with a resolution of 800x600). Following mydriasis with 1% Tropicamide, four 45° field nonstereoscopic images are captured by a professional medical photographer. The fields include the three used by the Liverpool Diabetic Eye Study7 plus the foveo-centric fourth field, to facilitate a clear macular view (Figure 1). Viewing the images on a Texet monitor 15″ with a resolution of 1280 × 1024, an experienced ophthalmologist grades them within a week. Referral to the eye service is according to the same criteria as for the optometric screening referrals. Quality assurance for the imaging system is secured by using the services of a professional photographer, an experienced intermediate-grade ophthalmologist who reads the images, and through special audit clinics. These are run by the consultant ophthalmologist examining a systematic sample (10%) of screening negative patients using slit-lamp biomicroscopy within 3 months of initial photography.
(c) The call–recall service for both screening modalities is administered by the Cheshire Health Agency based on the diabetes register. Patients who are currently attending the eye clinic are excluded from the call–recall system.
Hospital diabetic eye service (DES)—an experienced ophthalmologist examines and grades the referrals from both systems, using slit-lamp biomicroscopy. Appropriate treatment and/or follow-up is then arranged.
The study groups consist of the following. Optometry: (a) 769 patients screened by optometrists in the first year, (b) 51 patients referred to DES for STDR, (c) 55 patients referred for cataract/glaucoma with no STDR and in the first year.
Photography: (a) 874 patients screened by photography in its first year, (b) 68 screen negative patients from the audit clinic, and (c) 58 patients referred to the HDES following photography for STDR.
Statistical analysis was carried out using Instat® software on the Apple MacIntosh® computer. Two-sided P values, using Fisher's exact test, were calculated for referral diagnoses of background diabetic retinopathy and maculopathy for each screening model, and for false positive rates.
Results
A total of 6294 people (2.05% of the district population) were identified as having diabetes. Of these, 1312 (21%) have type I IDDM and 4982 (79%) have type 2 NIDDM. The whole population is predominantly Caucasian with only 1.1% being of ethnic origin.
Optometry model
A total of 14 optometrists carried out between eight and 50 screenings in the first year. A total of 1708 patients were invited to attend the accredited optometrists. In all, 769 patients attended, giving a compliance rate of 45%. There were 429 (55.7%) males and 340 (44.3%) females. Of the patients, 29 were less than 30 years, 273 were between 30 and 60 years, and 467 were over 60 years (1 : 9 : 16). The mean age was 62.8 years. In total, 591 patients had no retinopathy (76.8%). The distribution of diabetic retinopathy was as follows: 168 (21.8%) mild to moderate background retinopathy; eight (1%) severe including preproliferative diabetic retinopathy, five (0.7%) with proliferative retinopathy, and 29 (3.8%) patients with maculopathy (Table 1). Overall, 29 patients (3.8%) were referred directly to the diabetic eye clinic. In addition, 157 patients had a degree of cataract in either eye (20.4%) and glaucoma was diagnosed/suspected in 21 patients (2.7%). A total of 55 (7.1%) patients were referred for cataract and glaucoma through the general practitioner.
Digital photography
Of the 1748 invitations sent out for retinal photography, 874 attended giving a compliance rate of 50%. There were 456 (52.1%) males and 418 females (47.9%). The age distribution was: 36 patients less than 30 years, 314 patients between 30 and 60 years, and 530 patients over 60 years (1 : 9 : 15) with a mean age of 61.2 years. In sum, 682 patients had no retinopathy (78.1%). The distribution of diabetic retinopathy was as follows: 149 (17.1%) mild to moderate background retinopathy; 15 (1.8%) severe background retinopathy including pre-proliferative changes; two (0.2%) with proliferative retinopathy; 12 (1.7%) with maculopathy (Table 1); 37 (4.2%) patients were referred to the hospital diabetic eye service.
Although the two samples were not specifically age and sex matched, the male : female ratio and the age distribution of the two groups are similar. A significantly higher proportion of patients were diagnosed with very mild to moderate background retinopathy by the optometrists (P=0.03). Diabetic maculopathy, too, was recorded in a significantly larger group by the optometrists (P=0.02). There was no statistically significant difference in the overall rate of referrals by both models.
Sensitivity and specificity of the two systems could not be determined because of the lack of funding for examining the whole study cohort. A limited exercise has been carried out by extrapolating the findings at the DES and the Audit Clinic.
Hospital Diabetes Eye Service
Cases were classified as true positives if they satisfied the criteria for STDR, when examined in the DES.
In all, 51 consecutive patients seen from the optometrists were seen. The findings of the ophthalmologist were compared with the referral record of the optometrist. Of these, 13 were deemed unjustified referrals (Table 2). The records of the 55 patients referred for cataract/glaucoma, with apparently no STDR, were compared with findings in the eye clinic. One patient had severe background diabetic retinopathy. This was classified as false negative and the ratio of 1 : 54 was applied to the screen negative patients as a whole. The sensitivity and specificity for STDR was 75 and 98%, respectively (Table 3).
The 58 patients referred to the DES from the photography model were assessed. Referral was deemed unnecessary in 10 cases (Table 2). To determine sensitivity and specificity, we included 68 patients seen in the audit clinic. One patient had maculopathy—STDR (false negative). This ratio of 1 : 67 was applied to the whole screened population. The sensitivity and specificity for STDR were 80 and 98%, respectively (Table 4).
There was no statistical difference in the confirmed cases of maculopathy between the two screening modules. Comparison of other features/stages of diabetic retinopathy, too, did not show any statistically significant differences.
Costing
The cost of setting up each system is shown in Table 5. The cost of screening per patient is arrived at by dividing the total cost by the number of patients actually screened in the first year. The cost effectiveness is calculated by dividing the total cost by the numbers of true positives (test positives × sensitivity).
Optometric screening
Cost per screened case=cost of call–recall service per patient+optometry fee per patient=6550/769+£15.48=£23.99. Cost effectiveness for optometry=total cost/true positives=£18 454/22=£839.
Digital photography
Cost per actual patient screened=£25 599.30/874=£29.29. Cost effectiveness for digital photography=£25 599/30=£853.
Discussion
The prevalence of diabetes is 2.05%, comparing well with other reports.7,8,9,10 The overall prevalence of diabetic retinopathy in the two screened models varied from 21 to 24%. The prevalence in other studies from the UK has been reported as 30.3% in Exeter11 (EDRS), 40.4% in the Liverpool Diabetic Eye Study12 (LDES), 41% in insulin-requiring subjects,13 and 50% in noninsulin-requiring diabetics14 in the Melton Mowbray Study (MMS). The prevalence of maculopathy has been cited as 5–14%, severe preproliferative and proliferative retinopathy 1.1–9%.11,12,13,14 Our equivalent figures are 3.8% for maculopathy and 0.8% for proliferative retinopathy. The ethnic mix in our population is similar to the other groups. The lower figures for overall retinopathy and STDR must be explained by other factors. Firstly, we have excluded from the screening programmes all patients already attending the Diabetic Eye Clinic in the hospital. The three other studies all included, in their sample, patients already under the care of hospital diabetic eye specialists (up to 30% in MMS.14) Secondly, our programmes were established later than the others (Melton Mowbray, 1987; Exeter 1992; Liverpool Diabetic Eye Study, 1992) and hence were probably influenced by changing clinical practice with regard to glycaemic control. This effect could also explain to some degree the difference in our own two models with the optometry model being established 3 years later. We have been unable to access the biochemical details of all our patients to substantiate this theory. The existence of a good opportunistic screening prior to our systematic screening programmes may have also helped. Finally, some authors have suggested that lower prevalence rates could be a result of poor compliance.12
Compliance with both screening models in their first respective years of operation was equally poor. Compliance in the first year of other programmes has varied from 80 to 100%.8,11 On review, the DNA rate for the HESP had dropped to 15% by October 2000 (year 2). This increase in compliance is in contradistinction to the Exeter study, where DNA rates actually worsened over successive cycles.11 Improved coverage rather than minor differences in modes of screening had been suggested as the most economical way forward.15 A model in which a mobile screening unit visits inner city community clinics and performs mydriatic 35 mm colour photography has been well described.16 Compliance in the fifth year of its operation was 80%.7 Digital photography ideally should have as little transportation or movement as possible to avoid damaging the delicate optics and computer equipment.17
Optometric screening appeared to detect significantly higher rates of early retinopathy as well as maculopathy. Comparison of other features/stages of diabetic retinopathy did not show any differences. Although of little significance to the ophthalmologist, picking up early background retinopathy signals the need for tighter systemic control by the physician.3 The high proportion of unjustified referrals represents, in part, an initial ‘play safe’ attitude by the screener/reader. The sensitivity of 75% and specificity of 98% are similar to other optometry programmes.8,18 The use of slit-lamp biomicroscopy resulted in better sensitivity than the 65% reported using direct ophthalmoscopy.7 We agree with nsc that there is a lack of a hard record for quality assurance and/or for monitoring progressive change. Also, the critical size of the caseload below which practitioners may be unable to maintain their skills adequately is not known. In the first year, the number of patients screened per optometrist varied from eight to 50.
The sensitivity and specificity of digital photographic screening for diabetic retinopathy have been favourably compared with 35 mm colour photographic screening.19 The sensitivity of 80% and specificity of 98% in our system are similar to other reports.20,21,22 We are slightly concerned by the apparent failure of the digital system to pick up subtle macular changes, although there was no statistical difference in the confirmed cases of maculopathy between the two screening modules (ie they were equally specific). Better resolution and the use of stereoimages and oral fluorescein angiography may increase the detection of subtle macular oedema.7,23 Digital photography has an advantage over optometric screening in that there is a hard record, making quality assurance easier. Digital images also offered us the ease of acquisition, storage and transfer between screener/reader and treating ophthalmologist.
In our study, in their first respective years of operation, the cost per screened patient was £23.99 for optometry vs £29.29 for digital photography. The estimated cost of £23 per screened patient in the nsc model5 and the Liverpool Diabetic Eye Study24 is remarkably similar to our figure. We have based cost effectiveness, in the first year, on true positives and found the two models to be comparable (£839 for optometry vs £853 for digital). The nsc has arrived at a figure of £1370 for cost effectiveness. This, however, includes the cost of being seen by the ophthalmologist, laser treatment, follow-up, etc. If the costing was to be stopped at the stage of referral, the equivalent cost effectiveness figure is £270. This is in line with the Liverpool Diabetic Eye Study, where the cost effectiveness for systematic 35 mm photographic screening in its fifth year was £209.24 The nsc has estimated the cost effectiveness based on 8.5% referral and a compliance of 85% in the first year. Our referral rate from both screening programmes was 4%. We would explain this on the basis of previous efficient opportunistic screening and a systematic exclusion from the call–recall system of all diabetic patients attending any eye clinic (see above). Our revised cost effectiveness based on 85% attendance, a referral rate of 8.5%, and the current sensitivity would be as follows.
Optometry
Numbers of true positives=Compliance × total invites × referral rates × sensitivity=85% × 1708 × 8.5% × 75%=92.
Total cost=fee per patient+call–recall=1451(85% of 1708) × £15.48+£6550=£28 961
Cost effectiveness=Total cost/true positives=28 961/92=£315.
Digital photography
Numbers of true positives=compliance × total invites × referral rates × sensitivity=85% × 1748 × 8.5% × 80%=95.
Cost effectiveness=Total cost/true positives=£25 599/95=£269.
Thus, cost effectiveness improves substantially in both systems both by increased coverage and by increased referral rates. Poor compliance has a disproportionate bearing on the imaging programme as the fixed costs (including the capital outlay for the lease of the camera) are greater. Conversely, cost per screen as well as cost per true positive case (cost effectiveness) in the imaging model has the greater potential to come down as the coverage improves.
In conclusion, an optometry system can pick up minimal background retinopathy, highlighting the need for tight systemic control and secondary prevention. The imaging system is not always able to detect subtle retinopathy but is equally specific in identifying sight-threatening disease. Cost effectiveness was poor in both models as a reflection of poor compliance rates in the first year. Cost effectiveness of the imaging model should further improve with falling costs of imaging systems. Until then, it is essential to continue any existing well-coordinated optometry model.
References
Evans J . Causes of Blindness and Partial Sight in England and Wales 1990–1991. HMSO: London, 1995.
Diabetic Retinopathy Screening Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of diabetic retinopathy (DRS) findings. DRS Report No. 8. Ophthalmology 1991; 88: 583–600.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes and progression of long term complication in insulin dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986.
Baker R, Grimshaw G, Thompson JR, Wilson A . Services for diabetic retinopathy in England and Wales: a survey of ophthalmologists. Pract Diabetes Int 1989; 16(2): 33–34.
http://www.diabetic-retinopathy.screening.nhs.uk/recommendations.html.
British Diabetic Association. Practical guidance: initiating a district optometry screening programme for diabetic eye disease. A British Diabetic Association Report. September 1997
Harding SP, Broadbent DM, Neoh C, White MC, Vora J . Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ 1995; 311: 1131–1135.
Prasad S, Kamath GG, Jones K, Clearkin LG, Phillips RP . Effectiveness of optometrist screening for diabetic retinopathy using slit-lamp biomicroscopy. Eye 2001; 15: 595–601.
Wells S, Bennett I, Holloway G, Harlow V . Area widediabetes care: the Manchester experience with primary health care teams 1991–1997. Diabetic Med 1998; 15 (Suppl 3): S49–S53.
Siann T, Duncan EM, Sullivan F, Mathews D, Cromie DT . Area-wide diabetes care: the Lanarkshire experience with primary health care teams 1994–1997. Diab Med 1998; 15 (Suppl 3): 54–57.
Ling R, Ramsewak V, Taylor D, Jacob J . Longitudinal study of a cohort of people with diabetes screened by the Exeter Diabetic Retinopathy Screening Programme. Eye 2002; 16: 140–145.
Broadbent DM, Scott JA, Vora JP, Harding SP . Prevalence of diabetic eye disease in an inner city population: the Liverpool Diabetic Eye Study. Eye 1999; 13: 160–165.
Sparrow JM, McLeod BK, Smith TD, Birch MK, Rosenthal AR . The prevalence of diabetic retinopathy and maculopathy and their risk factors in the non-insulin treated diabetic patients of an English town. Eye 1993; 7: 158–163.
Mcleod BK, Thompson JR, Rosenthal AR . The prevalence of retinopathy in the insulin-requiring diabetic patients of an English country town. Eye 1988; 2: 424–430.
Thompson JR, Grimshaw GM, Wilson AD, Baker R . Screening for diabetic retinopathy: a survey of health authorities during a period of transition. J Eval Clin Practice 1999; 5(1): 81–85.
Owens DR, Gibbins RL, Kohner E, Grimshaw GM, Greenwood R, Harding SP . Diabetic retinopathy screening, 2000 Diabetes UK. Diabetic Med 2000; 17: 493–494.
Hildred RB . Alternative imaging in ophthalmology. Part 3: Retinal photography and diabetes in primary care. Eye News 2001; 7(6): 12–20.
Burnett S, Hurwitz B, Davey C, Ray J, Chaturvedi N, Salmazmann J et al. The implementation of prompted retinal screening for diabetic eye disease by accredited optometrists in an inner-city district of North London: a quality of care study. Diabetic Med 1998; 15: 238–243.
George LD, Halliwell M, Hill R, Adlington SJ, Lusty J et al. A comparison of digital retinal images and 35 mm colour transparencies in detecting and grading diabetic retinopathy. Diabetic Med 1998; 15: 250–253.
Buxton MJ, Sculpher MJ, Ferguson BA, Humphreys JE, Altman JFB, Spiegelhalter DJ et al. Screening for treatable diabetic retinopathy: a comparison of different methods. Diabetic Med 1991; 8: 371–377.
Gibbins RL, Owens DR, Allen JC, Eastman L . Practical application of the European Field Guide in screening for diabetic retinopathy by using ophthalmoscopy and 35 mm retinal slides. Diabetologia 1998; 41: 59–64.
O'Hare JP, Hopper A, Madhaven C, Charny M, Purewal TS, Harney B et al. Adding retinal photography to screening for diabetic retinopathy: a prospective study in primary care. BMJ 1996; 312: 679–682.
Newsom R, Moate B, Casswell T . Screening for diabetic retinopathy using digital colour photography and oral fluorescein angiography. Eye 2000; 14: 579–582.
James M, Turner DA, Broadbent DM, Vora J, Harding SP . Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ 2000; 320; 1627–1631.
Acknowledgements
This material was presented as a poster at the Oxford Eye Meeting, July 2002. We are indebted to Gaynor Shawcross, medical photographer and all accredited optometrists. We hold no proprietary interest in the imaging system mentioned in the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, K., Palimar, P., Sen, S. et al. Comparison of optometry vs digital photography screening for diabetic retinopathy in a single district. Eye 18, 3–8 (2004). https://doi.org/10.1038/sj.eye.6700497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700497
Keywords
This article is cited by
-
Prevalence and associated factors of vision loss in the South African National Health and Nutrition Examination Survey (SANHANES-1)
BMC Ophthalmology (2021)
-
Systematic review and meta-analysis of diagnostic accuracy of detection of any level of diabetic retinopathy using digital retinal imaging
Systematic Reviews (2018)