Abstract
Purpose To report the late presentation of an allergic reaction to brimonidine tartrate 0.2% associated with an elevation of intraocular pressure.
Methods During a 6-month period six Caucasian patients (three were male), with primary open angle glaucoma (POAG) or ocular hypertension, with an allergic reaction to brimonidine tartrate eye drops were identified. Brimonidine was initiated as additional medical therapy in four patients and monotherapy in two patients. The median age of the patients was 67 years (range 57–73 years).
Results There were nine eyes with a follicular conjunctivitis; three patients received brimonidine in one eye only. In two patients an additional redness of the periocular skin was present. The median duration on brimonidine therapy before the onset of the allergic reaction was 12 months (range 5–15 months). The median intraocular pressure (IOP) before the onset of the allergy was 18 mmHg (range 16–21 mmHg). There was a significant elevation of IOP at the time of the allergy with a median IOP of 28 mmHg (range 20–44 mmHg) (P = 0.007, Wilcoxon sign rank test). The cessation of brimonidine allowed the resolution of the allergic reaction. The intraocular pressure was then controlled with alternative medication in eight eyes. One patient went on to have filtering surgery.
Conclusions A delayed hypersensitivity reaction to brimonidine tartrate eye drops resembles a viral follicular conjunctivitis. It is imperative that it is recognised as such, as it may occur many months after brimonidine is initiated. This allergy has been found to be associated with a loss of control of the IOP. Though this is a small cohort of patients, it is not unreasonable to suggest that patients on brimonidine eye drops should be instructed to report promptly to their ophthalmologist the onset of redness of their eyes so that their glaucoma control may be reassessed.
Similar content being viewed by others
Introduction
Brimonidine tartrate is a highly selective alpha 2 adrenoreceptor agonist.1 Clinical trials have proved it to be an effective drug for treating chronically elevated IOP and the prophylactic treatment of acute pressure rises.2 Its hypotensive action is primarily attributed to aqueous suppression, though an uveoscleral outflow has also been suggested.3 Its long-term efficacy in lowering intraocular pressure is comparable to that of timolol eye drops4 but without the adverse cardiopulmonary side effects associated with the latter.5 Brimonidine is increasingly being used as a first line drug in primary open angle glaucoma,6 especially when beta-blockers are contraindicated.
Topical therapy with brimonidine tartrate is well tolerated; its adverse events are reported to be mild to moderate in nature.2 These include dryness of the mouth, fatigue-drowsiness, headache and an allergic reaction.4 Apart from ocular allergy the above adverse events are not statistically different from those patents on beta blockers.4,7
Ocular allergy associated with conjunctival follicles occurs in 4.8–9% of patients on brimonidine tartrate and up to 15% of patients may discontinue the drug due to an allergic reaction.4,8 This allergic reaction has been reported to occur within the first 6–9 months.
We report the late presentation of ocular allergy with the use of brimonidine tartrate associated with an elevated intraocular pressure in six patients.
Patients and methods
During a 6-month period six patients with an allergic reaction to brimonidine tartrate were identified. Five patents had POAG and one had ocular hypertension. Three patients presented acutely before their next follow-up appointment and three patients were seen at their routine follow-up appointment. The patients who presented acutely were treated with brimonidine in one eye only and had a unilateral allergic response. Patients reviewed at their routine follow-up had a bilateral allergic reaction to brimonidine instilled in both eyes. The median age of the patients was 67 years (range 57–73 years). There were three male and three female patients with a diagnosis of primary open angle glaucoma (POAG). Brimonidine tartrate 0.2% (Alphagan) twice a day was used as monotherapy in two patients. It was used in addition to betaxolol 0.5% twice a day in three patients and in addition to betaxolol 0.5% twice a day with dorzolamide 2% twice a day in one patient. Visual fields were performed with a Humphrey Field Analyser, program 24–2.
Results
The allergic reaction consisted of a follicular conjunctivitis, which was unilateral in three patients who instilled brimonidine in one eye only. Three patients had an additional swelling and redness of the periocular skin. The median IOP, before brimonidine was started, in the eyes that developed an allergic reaction was 28 mmHg (range 23–30 mmHg). The median IOP of the eyes while on brimonidine before the development of the allergic reaction was 18 mmHg (range 16–21 mmHg). The median IOP in the eyes (nine eyes) at the time of the allergic reaction was 28 mmHg (range 20–44 mmHg). This difference was statistically significant (P = 0.007, Wilcoxon sign rank test). All the patients had continued to use all their ocular hypotensive medication, including brimonidine, until the diagnosis of the allergic reaction was established (Table 1).
When brimonidine was stopped, the intraocular pressure was controlled with a combination of betaxolol 0.5% twice a day, latanoprost 0.005% once a day in two patients, latanoprost 0.005% alone in one patient, dorzolamide 2% three times a day in one patient and betaxolol 0.5% and dorzolamide 2% twice a day in one patient. One patient had a trabeculectomy due to failure of control of IOP with alternative medical therapy. The allergic reaction resolved within a week of cessation of brimonidine eye drops. Three representative cases are presented.
Case 2
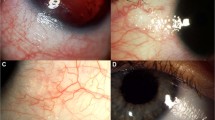
A 68-year-old Caucasian male was referred from his optician with high IOP. His visual acuity was 6/6 with an IOP of 30 mmHg in his right and left eye. He had open angles on gonioscopy. He had a CDR of 0.8 on the right and 0.9 on the left with a left relative afferent pupillary defect. He was started on brimonidine in both eyes. His left eye underwent a trabeculectomy as a target IOP was not achieved with medical therapy. The intraocular pressure was controlled with brimonidine in the right eye with an average IOP of 18 mmHg and stable visual fields. His left eye was on no medication with an IOP of 18 mmHg and a functioning filtering bleb. He presented 15 months later with a 2-week history of an acute red right eye. On examination his right eye had a follicular conjunctivitis (Figure 1) with an IOP of 44 mmHg in his right eye and 18 mmHg in his left eye with quiet anterior chambers. The follicular conjunctivitis resolved within a week of cessation of brimonidine eye drops. The IOP was subsequently controlled in the right eye with betaxolol 0.5% twice a day and latanoprost 0.005% once a day.
Case 3
A 71-year-old Caucasian female was referred to the eye clinic from her optician with a high IOP in her left eye. On examination her visual acuity was 6/5 in the right eye and 6/6 in the left eye. Her IOP was 20 mmHg in the right eye and 30 mmHg in the left eye, with wide-open angles on gonioscopy. The right eye had a CDR of 0.1 and the left a CDR of 0.8 with a normal right visual field. The IOP was controlled with timolol 0.25% and dipivefrin 0.1% twice a day in her left eye. This was substituted with brimonidine as the patient experienced breathlessness on the above medication. The IOP was an average of 16 mmHg on brimonidine eye drops.
In between her routine 6-monthly appointments she visited her general practitioner with an acute redness of her left eye, this was treated with sodium fusidate, however when the redness failed to resolve she was referred to the eye clinic 4 weeks later. On examination she had a puffy left lower lid with a follicular conjunctivitis. The IOP was 28 mmHg with a quiet anterior chamber. The conjunctivitis resolved with the cessation of brimonidine. The intraocular pressure was 26 mmHg and she was rechallenged with brimonidine when the allergy recurred with an elevated intraocular pressure of 30 mmHg. The left eye underwent a trabeculectomy and achieved good IOP control with a functioning filtering bleb at 3 months follow-up.
Case 6
A 66-year-old female was referred to the eye clinic from her optician with high IOP. She had chronic obstructive airways disease. Her visual acuity was 6/6 in her right and left eye, with presenting IOP of 30 mmHg in the right eye and 28 mmHg in the left eye, the anterior chamber angles were wide open gonioscopy. She had a 0.5 and 0.3 CDR in the right and left eye respectively. Brimonidine eye drops were started, which brought the IOP down to 16 mmHg in both eyes. At a routine follow-up appointment 12 months from the initiation of the above drops, she had a redness of the periocular skin (Figure 2) with a bilateral follicular conjunctivitis. She volunteered that her eyes had been red over the preceding 2 months and that there had been no response to the sodium fusidate prescribed by the general practitioner. Her IOP measured 24 mmHg in the right eye and 20 mmHg in the left eye. The anterior chambers were quiet. The follicular conjunctivitis resolved a week after the brimonidine eye drops were stopped. Her IOP was controlled with dorzolamide eye drops three times a day.
Discussion
Six patients developed an allergic reaction to brimonidine tartrate 0.2% eye drops instilled twice a day, this consisted of a follicular conjunctivitis and a swelling and redness of the periocular skin. The patients were diagnosed as having an infective conjunctivitis by their general practitioners and were treated with antibacterial drugs. When there was no response to treatment they were referred to the eye department. Hence there was a delay, which ranged from 2 to 8 weeks (median 6 weeks) before a diagnosis of an allergic conjunctivitis was established. This delayed hypersensitivity response occurred up to 15 months (range 5–15 months, median 12 months) after the drops were started. At the time of the allergic reaction while the patients were still using the drops, the IOP was found to be significantly elevated with a median of pressure of 28 mmHg (range 20–44 mmHg) (P = 0.007).
The allergic reaction resembles a viral conjunctivitis without corneal involvement; hence a high index of suspicion should be entertained when a patient with glaucoma on brimonidine presents with a follicular conjunctivitis. The patients with an allergic reaction do not develop a keratitis as seen with viral disease. It has been reported that this allergic reaction which occurs in 4.8–9% of patients on brimonidine eye drops is usually seen within the first 9 months.4,8 In our series of patients a much later presentation was seen in four of the six patients. This may be due to the fact that the studies reporting the delayed hypersensitivity reaction were followed up for 12 months.4,7,8 It is possible that with a longer duration of follow-up we may uncover more cases developing an allergic reaction to brimonidine.
The fact that the allergic response may be associated with an elevated IOP or loss of a previously controlled IOP is of more serious consequence. The median IOP elevation was 28 mmHg, with two patients with an IOP above 40 mmHg. Patients with an allergic reaction may wait till their next appointment before advice is sought, and this may jeopardise an already compromised optic disc. We are unaware of previous studies reporting this association.
The mechanism of pressure elevation may simply represent a failure of the ocular hypotensive effect with the onset of the allergic reaction, however this will not account for the IOP being higher than the presenting IOP. There was no evidence of an iritis at the time of the allergic reaction; hence a hypertensive uveitis is discounted. None of the patients received steroids as part of the treatment of the conjunctivitis, which would eliminate a steroid related elevated intraocular pressure. The possibility of an increased episcleral pressure associated with the vascular congestion is a plausible explanation, which is supported by the fact that the IOP is controlled with alternative medication when the conjunctivitis resolves without the use of topical steroids.
This study suffers from the small number of patients presented. It is not known how many patients with an allergic reaction will develop a high IOP. A prospective study is now underway to identify all patients with a delayed hypersensitivity to brimonidine tartrate eye drops who may also have a high IOP.
Conclusions
An allergic reaction to brimonidine tartrate 0.2% eye drops resembles a viral conjunctivitis (without corneal involvement) and may delayed up to 15 months after treatment is started. This may be associated with a high IOP. It is imperative therefore that an allergic reaction to the drug is recognised promptly and the IOP is assessed. Failure to do so may allow an uncontrolled IOP to jeopardise an already compromised optic disc.
References
Burke J, Schwartz M . Preclinical evaluation of brimonidine. Surv Ophthalmol 1996; 41 (suppl 1): S9–S18
Walters TR . Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: a review of safety, efficacy, dose response, and dosing studies. Surv Ophthalmol 1996; 41 (Suppl 1): S19–S26
Toris CB, Gleason ML, Camras CB . Effects of brimonidine on aqueous humour dynamics in human eyes. Arch Ophthalmol 1995; 113: 1514–1517
LeBlanc RP for the Brimonidine Study Group 2. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Ophthalmology 1998; 105: 1960–1967
Stewart WC, Garrison PM . B-Blocker-induced complications and the patient with glaucoma. Newer treatments to help reduce systemic adverse events. Arch Intern Med 1998; 158: 221–226
Wilensky JT . The role of brimonidine in the treatment of open angle glaucoma. Surv Ophthalmol 1996; 41 (Suppl 1): S3–S7
Serle JB the Brimonidine Study Group III. A comparison of the safety and efficacy of twice daily brimonidine 0.2% versus betaxolol 0.25% in subjects with elevated intraocular pressure. Surv Ophthalmol 1996; 41 (Suppl 1): S39–S47
Schuman JS, Horwitz B, Choplin NT, David R, Albracht D, Chen K the Chronic Brimonidine Study Group. A 1-year study of brimonidine twice daily in glaucoma and ocular hypertension. Arch Ophthalmol 1997; 115: 847–852
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watts, P., Hawksworth, N. Delayed hypersensitivity to brimonidine tartrate 0.2% associated with high intraocular pressure. Eye 16, 132–135 (2002). https://doi.org/10.1038/sj.eye.6700053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700053