Abstract
Background:
The combination of chemotherapy with the vascular endothelial growth factor (VEGF) antibody bevacizumab is a standard of care in advanced colorectal cancer (CRC). However, biomarkers predicting outcome of bevacizumab-containing treatment are lacking. As angiopoietin-2 (Ang-2) is a key regulator of vascular remodelling in concert with VEGF, we investigated its role as a biomarker in metastatic CRC.
Methods:
Serum Ang-2 levels were measured in 33 healthy volunteers and 90 patients with CRC. Of these, 34 had metastatic disease and received bevacizumab-containing therapy. To determine the tissue of origin of Ang-2, quantitative real-time PCR was performed on microdissected cryosections of human CRC and in a murine xenograft model of CRC using species-specific amplification.
Results:
Ang-2 originated from the stromal compartment of CRC tissues. Serum Ang-2 levels were significantly elevated in patients with metastatic CRC compared with healthy controls. Amongst patients receiving bevacizumab-containing treatment, low pre-therapeutic serum Ang-2 levels were associated with a significant better response rate (82 vs 31%; P<0.01), a prolonged median progression-free survival (14.1 vs 8.5 months; P<0.01) and a reduction of 91% in the hazard of death (P<0.05).
Conclusion:
Serum Ang-2 is a candidate biomarker for outcome of patients with metastatic CRC treated with bevacizumab-containing therapy, and it should be further validated to customise combined chemotherapeutic and anti-angiogenic treatment.
Similar content being viewed by others
Main
The overall survival (OS) time of patients with metastatic colorectal cancer (CRC) has increased owing to novel therapeutic strategies combining chemotherapy with monoclonal antibodies against vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR). Whereas mutations in the k-ras oncogene predict outcome to EGFR antibody treatment in patients with metastatic CRC (Lievre et al, 2006; Karapetis et al, 2008), equivalent biomarkers for the VEGF antibody, bevacizumab, are currently lacking (Jubb et al, 2006b; Sessa et al, 2008), mainly because the specific molecular determinants of clinical response and resistance to the drug are unknown. Similarly, there are currently no established biomarkers predicting outcome to chemotherapy in CRC (De Roock et al, 2009).
Originally, it was anticipated that traditional markers of tumour angiogenesis would predict outcome to bevacizumab. However, neither VEGF expression levels nor tumour microvessel density (MVD) were found to be predictive of treatment response, disease progression or death in CRC patients receiving chemotherapy plus the antibody (Jubb et al, 2006a). Newer studies evaluated alternative surrogates of neovascularisation such as circulating endothelial cells and phosphorylated VEGF receptor in cancers other than CRC (Murukesh et al, 2010). Results were promising, but assay requirements prevent widespread application in larger trials.
The therapeutic blockade of VEGF by bevacizumab in CRC patients induces complex changes in the stromal compartment of the tumour lesion, including the loss of chaotic microvessels, remodelling of the vascular wall and a reduction in the interstitial fluid pressure (Willett et al, 2004). Such stromal alterations are part of the vascular ‘normalisation’ process induced by bevacizumab and contribute to more efficient delivery of chemotherapeutic agents (Jain, 2005; Jain et al, 2006). Recently, dynamic contrast-enhanced magnetic resonance imaging was proposed to assess the extent of vascular normalisation and predict clinical outcome to VEGF-targeting treatment (Sorensen et al, 2009; Murukesh et al, 2010). However, molecular markers reflecting the normalisation status of the tumour vascular bed as part of the tumour stroma have not been explored in this regard so far.
The molecular alterations of tumour cells are traditionally regarded as the major determinants of clinical response to chemotherapy. However, the above observations suggest that the vascular microenvironment of tumour cells could be equally important for efficacious cytostatic treatment. Surrogates of vascular normalisation, therefore, might predict outcome to chemotherapy as well as to VEGF-targeting therapy.
Angiopoietin-2 (Ang-2) is an inhibitory ligand of the Tie-2 receptor that is stored in the Weibel–Palade bodies of endothelial cells (Fiedler et al, 2004) and disrupts the integrity of the blood vessel wall, thus counteracting vascular normalisation (Maisonpierre et al, 1997; Scharpfenecker et al, 2005; Falcon et al, 2009; Reiss et al, 2009). On the basis of this, we expected Ang-2 to be predominantly expressed in the stromal compartment of CRC, and hypothesised that low and high serum Ang-2 levels in patients with metastatic CRC would predict different outcomes to bevacizumab-containing treatment.
Materials and methods
Microdissection
For microdissection analysis, cyrosections were prepared from human adenocarcinoma samples. Discrete areas of tumour or stromal tissue were microdissected using a laser microbeam (P.A.L.M., Bernried, Germany). Microdissected tissue areas (each 100 μm in diameter, 30 per slide for stroma and tumour each) were laser catapulted into a microfuge tube.
Xenografts
Animal experiments were performed in accordance with the German animal protection law. The colon carcinoma cell line LS174T was purchased from ATCC (Manassas, VA, USA) to establish xenografts in nude mice. Cultured LS174T cells (5 × 106) were injected subcutaneously into the flank region of male BALB/cA nude mice (Taconic, Ry, Denmark). Animals were killed after tumour size had reached 10 mm in diameter.
Cell cultures
The colon carcinoma cell lines LS174T, HT29, DLD-1 and SW948 were cultured in VLE RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 10% FCS. Human umbilical vein endothelial cells were purchased from Promocell (Heidelberg, Germany) and cultured in endothelial cell growth medium (Promocell). All cell lines were maintained at 37°C and 5% CO2, except for one experiment involving exposure to controlled hypoxia (1% O2, 5% CO2, balanced N2) for 24 h.
Quantitative real-time PCR
The RNA was extracted from tissue microdissections using the NucleoSpin RNA isolation kit (Macherey-Nagel, Düren, Germany) and reverse transcribed using the RevertAid cDNA Synthesis Kit (Fermentas, St Leon-Rot, Germany). For gene expression analysis in xenografts, tumours were suspended in RNAlater (Qiagen, Hilden, Germany). The RNA was extracted as described above. Real-time PCR for Ang-2 was carried out using the LightCycler FastStart DNA Master Plus Mix (Roche, Basel, Switzerland). Results were normalised to a dilution series of pre-quantified pooled PCR products with the RelQuant Software (Roche). Primer sequences for human- and murine-specific amplification of Ang-2 and β-actin were as published (Thijssen et al, 2004). Universal GAPDH primers were as follows: Forward 5′-TGC(A/C)TCCTGCACCACCAACT-3′, Reverse 5′(C/T)GCCTGCTTCACCACCTTC-3′.
Western blot
For western blot (WB) analysis, cytosolic extracts of cultured cells were prepared as described previously (Kashkar et al, 2002). Equal amounts (100 μg) of protein were separated by SDS-PAGE, transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), and probed with Ang-2 antibody (F18, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Primary antibodies were detected using a horseradish-peroxidase-conjugated secondary antibody (1 : 2000; Dako, Hamburg, Germany) and visualised with the ECL system (Amersham Biosciences, Hamburg, Germany). Culture supernatants were analysed for Ang-2 protein concentrations using Quantikine Immunoassays (R&D Systems, Wiesbaden, Germany).
Clinical samples
A total of 90 patients with colorectal adenocarcinoma and 33 healthy volunteers were studied between September 2005 and November 2008. One cohort of 56 patients had newly diagnosed CRC of various stages (UICC I–IV). After obtaining informed consent, serum samples and tumour tissues were collected at the time of primary resection (sampling from September 2005 to August 2006). A second cohort of 34 patients had primary (n=25) or relapsed (n=9) CRC of advanced stage and received a combination treatment of bevacizumab and chemotherapy either in the context of a clinical trial (AIO trial KRK 0604, n=15) conducted by the Department of Internal Medicine of the University Hospital Bochum (Reinacher-Schick et al, 2008) or without participating in a clinical trial (n=19) at the Center of Integrated Oncology Cologne-Bonn. Following approval by the Ethics Committees, serum samples from these patients were taken prior to treatment (sampling from March 2006 to April 2008). Where available, paraffin blocks of tumour tissue were retrieved from the local pathology archives.
Demographical, clinical and histopathological baseline parameters were documented in all patients. For patients receiving bevacizumab-containing therapy, the clinical response after 2 months of treatment was assessed according to response evaluation criteria in solid tumours (RECIST). Patients were continuously monitored during the course of treatment and disease progression and deaths occurring during and after therapy were recorded.
Enzyme-linked immunosorbent assay
Quantikine Immunoassays (R&D Systems) were used to measure protein concentrations of Ang-2 and VEGF in serum samples according to the manufacturer's instructions.
Immunohistochemistry
Sections of paraffin tissue blocks of CRC specimens were processed for histological analysis as previously described (Eberhard et al, 2000). Immunohistochemistry (IHC) for Ang-2 was carried out with the following antibodies: MAB 0983 (R&D Systems), N18 and F18 (Santa Cruz Biotechnology). To assess non-specific antibody binding, Ang-2 antibodies were blocked by pre-incubation with recombinant Ang-2 (ratio 1 : 5, R&D systems) prior to incubation of histological sections. A biotinylated secondary antibody, streptavidin peroxidase complex, and diaminobenzidine as the substrate (Zymed, Carlsbad, CA, USA) were used to visualise binding of the primary antibody (followed by semiquantitative assessment of Ang-2 staining intensity). Tumour blood vessels were stained with the CD34 antibody QBEnd/10 (Novocastra Laboratories, Wetzlar, Germany) and the α-SMA antibody 1A4 (Dako). Microvessel density and pericyte coverage (PC) were assessed as described previously (Eberhard et al, 2000).
Statistical analysis
The SPSS 17 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Areas under receiver operating characteristic curves (AUROCs) were measured to assess the diagnostic performance of continuous test variables for treatment response as the target variable. Youden's index was calculated to determine cutoff values of test variables. Parameters others than survival were compared using the Mann–Whitney test and the χ2-test. Overall and progression-free survival (PFS) was calculated using the Kaplan–Meier method. The log-rank test was used to compare survival time periods between groups. A Cox regression model was applied for univariate and multivariate analysis to estimate hazard ratios. All statistical tests were two sided. Statistical significance was defined as P<0.05. Data analysis is reported according to REMARK guidelines (Mallett et al, 2010).
Results
Ang-2 is expressed in the stromal compartment of CRC but not in the tumour cells
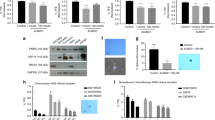
Extensive immunohistochemical analysis of Ang-2 with three frequently reported commercial Ang-2 antibodies produced ambiguous results concerning the tissue localisation in human CRC because of poor antibody specificity (Supplementary Figure 1). Therefore, to identify the tissue of origin of Ang-2 in CRC, laser-captured microdissection was used to isolate the tumour and stromal compartments from tissue sections of CRC patients for quantitative real-time PCR (Figure 1A). The Ang-2 mRNA was clearly detectable in the dissected stromal compartment, but not in the tumour cell compartment (Figure 1B).
Expression of Ang-2 in CRC. (A) Illustration of the steps involved in laser microdissection of cryosections of CRC patients to obtain stromal (S) and tumour (T) material for RT–PCR amplification. The top row shows capture of tumour and the bottom row capture of stroma from the same section. (B) Gel electrophoresis of end point RT–PCR amplification products of Ang-2 and β-actin on material of a whole tumour section (W) or microdissected stromal or tumour areas of sections of five different CRC patients showing Ang-2 expression in W and S, but not in T. β-Actin served as a control. The bar chart shows the relative quantitative real-time PCR of Ang-2 levels normalised to β-actin expression using the ΔCt method. Bars represent the means of five patients with standard errors showing significant Ang-2 expression (100%) in the tumour stroma, but undetectable in the tumour cells themselves (*). Some expression (<10%) is also seen when whole sections were amplified, owing to the Ang-2 expressing stromal compartment. (C) End point RT–PCR analysis of xenograft tumours of human LS174T cells in nude mice analysed by species-specific amplification for human (tumour) and murine (stromal) Ang-2. Xenograft tumours show stromal-derived murine Ang-2, but not tumour-derived human Ang-2. The GAPDH was used as a species independent control. Human umbilical vein endothelial cells (HUVEC) served as a positive control for human Ang-2.
Xenografts of CRC in nude mice were generated to further verify the stromal origin of Ang-2. In these animals, the stromal compartment is of murine origin and the tumour cell compartment is of human origin (LS174T colon carcinoma cells). Using species-specific RT–PCR, Ang-2 mRNA expression was found to be exclusively of stromal (murine) origin (Figure 1C). Correspondingly, no significant amount of Ang-2 protein was detectable in cytosolic extracts and culture supernatants of various colon carcinoma cell lines (LS174T, HT29, DLD-1 and SW948) cultured under normoxia or hypoxia to mimic the conditions inside the tumour (Supplementary Figure 2).
Serum Ang-2 levels are elevated in CRC patients with metastatic disease
Blood serum concentrations of Ang-2 were studied in a total of 90 patients with colorectal adenocarcinoma and 33 healthy volunteers. The patient demographics are summarised in Table 1. In UICC stage IV patients, serum Ang-2 levels were significantly higher compared with patients with UICC stage I–III or healthy controls (3.9 vs 2.3 ng ml−1, P=0.001 and 3.9 vs 2.4 ng ml−1, P=0.006, respectively) (Figure 2). In contrast, serum Ang-2 levels did not differ significantly between UICC stage I–III patients and controls.
Serum Ang-2 levels identify CRC patients of different clinical outcomes to bevacizumab-containing therapy
On enrolment, 34 patients with primary or relapsed CRC of UICC stage IV were treated with bevacizumab in combination with chemotherapy and followed for clinical outcome. Individual patient characteristics and outcomes are presented in Table 2. A total of 19 patients responded to treatment, whereas the rest had stable or progressive disease. The overall PFS was 12.1 months. After a median follow-up period of 16.6 months (range 4–26), the median OS had not been reached. No significant differences in survival times and response rates were found between different study sites, gender or age groups (Supplementary Table 1).
Pre-therapeutic serum Ang-2 concentrations in CRC patients ranged from 0.7 to 12.1 ng ml−1 (median: 3.5 ng ml−1) and did not correlate with other vascular markers such as serum levels of VEGF (median: 0.19 ng ml−1), tumour MVD (median: 42 per HPF) or PC (median: 52%). Measured serum Ang-2 and VEGF levels were unbiased by gender, age, ECOG status, treatment line or regimen and showed no correlation with the numbers of metastatic sites (Supplementary Table 2).
The AUROC was 0.77 for serum Ang-2 (95% CI: 0.61–0.94; P=0.008) when using treatment response as the outcome parameter. The AUROC for tumour MVD was 0.81, but statistical significance was marginal (95% CI: 0.53–1.00; P=0.03). For serum VEGF and PC, AUROC did not differ significantly from 0.50. Based on ROC analysis and Youden's index, a serum Ang-2 of 3.5 ng ml−1 was chosen as the cutoff value and patients were dichotomised into subgroups with low or high serum Ang-2 concentrations. Significantly more patients with low serum Ang-2 levels responded to treatment than patients with high serum Ang-2. The overall response rate (OR) in the two groups was 82 and 31% (P=0.005), respectively (Figure 3A). Mean serum Ang-2 concentrations were lower in treatment responders compared with non-responders (3.3 vs 5.8 ng ml−1; P=0.008). Disease control was significantly better in patients with low serum Ang-2 levels than in patients with high serum Ang-2 (median PFS: 14.1 vs 8.5 months; P=0.009) (Figure 3B). There was a 63% reduction in the hazard of progression for patients with low serum Ang-2 compared with those with high serum Ang-2 (HR 0.37; 95% CI: 0.17–0.80; P=0.01). Overall survival was remarkably prolonged in patients with low serum Ang-2 levels (median OS: not reached) compared with the group of patients with high serum Ang-2 (median OS: 16.2 months; P=0.004). Survival rates at 1.5 years were 94 versus 53% in the low and high serum Ang-2 group, respectively (Figure 3C), and the hazard of death in low Ang-2 patients was reduced by 91% compared with patients with high serum Ang-2 levels (HR 0.09; 95% CI: 0.01–0.70; P=0.02). Differences in survival remained significant when patients with primary and relapsed CRC were analysed separately (Supplementary Table 3), and in a multivariate analysis of variables with potential impact on OR, PFS and OS (gender, age, site, treatment regimen and treatment line), serum Ang-2 was confirmed as an independent prognostic marker for all three end points (P=0.003, P=0.005 and P=0.003, respectively). In contrast to serum Ang-2 levels, there was no significant association between OR, PFS or OS and low vs high serum VEGF or tumour PC, respectively, using cutoff values as determined by ROC analysis and Youden's index. Similarly, tumour MVD was not associated with these end points except for OR (Table 2, Figure 4).
Discussion
Bevacizumab is a VEGF-targeting antibody that is widely used in combination with chemotherapy to treat metastatic CRC (Cercek and Saltz, 2008; McCormack and Keam, 2008). Although much has been learned about its mechanisms of action, suitable biomarkers predicting patients who are likely to benefit from bevacizumab treatment remain elusive (Jubb et al, 2006b; Sessa et al, 2008; Murukesh et al, 2010). Expression levels of VEGF, in particular, are not predictive of outcome in CRC patients treated with bevacizumab (Jubb et al, 2006a). So far, the search for outcome predictors in CRC has failed for bevacizumab and antibody-free chemotherapeutic regimens alike (De Roock et al, 2009).
Although VEGF is primarily produced by tumour cells, its target structure is the tumour vasculature embedded in the stromal compartment, where the therapeutic effects of the antibody involve extensive changes such as blood vessel pruning and reorganisation of the chaotic tumour vasculature (Willett et al, 2004). Thus, stromal factors controlling the responsiveness of blood vessels to VEGF withdrawal rather than determinants of VEGF availability are attractive candidates as outcome predictors for bevacizumab treatment. Potentially, stromal factors are also outcome predictors of chemotherapy, because the delivery of cytostatic drugs to tumour cells is controlled by the vascular tumour microenvironment.
Angiopoietin-2 has been proposed as a gatekeeper of VEGF function and vascular remodelling. (Hanahan, 1997; Augustin et al, 2009). We here identified Ang-2 as a stromal factor in CRC. In tumour lesions of CRC patients and in a murine xenograft model of CRC, Ang-2 mRNA was expressed exclusively in the tumour stromal compartment, but not in the tumour cell compartment itself. Although these findings are at odds with previous immunohistochemical studies reporting Ang-2 expression in the tumour cell compartment of CRC (Ochiumi et al, 2004; Ogawa et al, 2004; Chung et al, 2006; Gu et al, 2006), the published immunohistochemical data should be interpreted with caution owing to the limited specificity of the available antibodies. Indeed, careful analysis of the tissue localisation of Ang-2 expression in cancer entities and tumour models other than CRC has called into question the tumour cell origin of Ang-2 (Zhang et al, 2003).
To further elucidate the clinical impact of stromal-derived Ang-2, we measured serum Ang-2 concentrations in CRC patients. Serum Ang-2 levels were significantly elevated in patients with metastatic disease. Indeed, Ang-2 has been shown to promote metastatic growth (Imanishi et al, 2007). Although one can only speculate as to what extent the stromal expression of Ang-2 contributes to serum Ang-2 concentrations in patients with advanced CRC, serum levels of Ang-2 in such patients were found to be significantly higher than in healthy individuals. It seems likely, therefore, that serum Ang-2 levels reflect the level of Ang-2 expression in the tumour stromal compartment.
Elevated serum concentrations of Ang-2 have also been reported for patients with cancers other than CRC, such as non-small-cell lung cancer and melanoma, in which high serum Ang-2 levels correlate with disease stage and poor OS (Park et al, 2007; Helfrich et al, 2009). However, the relationship between serum Ang-2 levels and clinical outcome in patients treated with VEGF-targeting drugs and chemotherapeutic agents has not been explored before, and this study is the first to investigate the impact of pre-therapeutic serum Ang-2 concentrations on the clinical outcome in patients with metastatic CRC under bevacizumab-containing therapy. Compared with high serum Ang-2 levels, low serum Ang-2 was associated with an outstanding response rate (>80%), better disease control and excellent OS (>90% after 18 months). In accordance with previous reports (Jubb et al, 2006a), VEGF and tumour MVD were not similarly correlated to these end points. Similarly, the pericyte content of CRC was not linked to treatment outcome and did not correlate with Ang-2 serum concentrations, indicating that serum Ang-2 is probably not simply a surrogate of blood vessel morphology.
On the basis of experimental models, Ang-2 has been described as an opponent of vascular normalisation that prevents blood vessels from becoming structurally and functionally stabilised (Maisonpierre et al, 1997; Scharpfenecker et al, 2005; Falcon et al, 2009; Reiss et al, 2009). Conceivably, normalisation of tumour vessels by bevacizumab-mediated blockade of VEGF may be more difficult to achieve and chemotherapeutic drugs cannot be delivered appropriately to the tumour cells when Ang-2 serum levels are high. From a mechanistic point of view, the observation that patients with low serum Ang-2 were most likely to benefit from treatment with respect to major clinical end points supports such a biological role of Ang-2. From a clinical perspective, our observations suggest that serum Ang-2 could hold promise as a predictive biomarker allowing bevacizumab-containing treatment to be customised in CRC patients.
Having analysed a heterogeneous patient cohort of moderate size, our study is not without limitations. Nevertheless, subgroup analyses of known prognostic factors in CRC (for example, age, ECOG) showed no evidence that outcome by Ang-2 was biased by those factors. The major and novel finding of this study is that pre-therapeutic serum Ang-2 not only predicted OS in the study population but was also predictive of therapeutic end points (PFS, response rate), suggesting that Ang-2 is a stromal determinant of both resistance and response to bevacizumab-containing therapy. As this is a single-arm study, it remains undecided whether serum Ang-2 is a specific outcome predictor for bevacizumab or chemotherapy or for the combination of both. Irrespective of its specific predictive properties, however, measurements of pre-therapeutic serum Ang-2 could be valuable. For example, CRC patients awaiting secondary resection of metastases could be stratified by serum Ang-2 levels into risk groups. Whereas patients with low serum Ang-2 are likely to benefit from neoadjuvant bevacizumab-containing treatment, patients with high serum Ang-2 could require escalation of chemotherapy or use of other biologicals such as EGFR antibodies in patients with wild type k-ras oncogene or Ang-2 inhibitors that are currently in clinical development (Hu and Cheng, 2009). Although definitive judgement on the role of Ang-2 as a specific biomarker of outcome to bevacizumab in CRC or other cancers will require analysis of large numbers of blood samples from phase III clinical trials comparing bevacizumab-containing therapy with chemotherapy alone, the promising results of this study should encourage researchers to further investigate the predictive value of Ang-2 in cancer treatment.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177
Cercek A, Saltz LB (2008) First-line treatment of patients with metastatic colorectal cancer: an overview of recent data on chemotherapy plus targeted agents. Clin Colorectal Cancer 7 (Suppl 2): S47–S51
Chung YC, Hou YC, Chang CN, Hseu TH (2006) Expression and prognostic significance of angiopoietin in colorectal carcinoma. J Surg Oncol 94: 631–638
De Roock W, Biesmans B, De Schutter J, Tejpar S (2009) Clinical biomarkers in oncology: focus on colorectal cancer. Mol Diagn Ther 13: 103–114
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG (2000) Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 60: 1388–1393
Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM (2009) Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol 175: 2159–2170
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156
Gu J, Yamamoto H, Ogawa M, Ngan CY, Danno K, Hemmi H, Kyo N, Takemasa I, Ikeda M, Sekimoto M, Monden M (2006) Hypoxia-induced up-regulation of angiopoietin-2 in colorectal cancer. Oncol Rep 15: 779–783
Hanahan D (1997) Signaling vascular morphogenesis and maintenance. Science 277: 48–50
Helfrich I, Edler L, Sucker A, Thomas M, Christian S, Schadendorf D, Augustin HG (2009) Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res 15: 1384–1392
Hu B, Cheng SY (2009) Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep 11: 111–116
Imanishi Y, Hu B, Jarzynka MJ, Guo P, Elishaev E, Bar-Joseph I, Cheng SY (2007) Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res 67: 4254–4263
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62
Jain RK, Duda DG, Clark JW, Loeffler JS (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 3: 24–40
Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, Hillan KJ, Koeppen H (2006a) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24: 217–227
Jubb AM, Oates AJ, Holden S, Koeppen H (2006b) Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer 6: 626–635
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359: 1757–1765
Kashkar H, Kronke M, Jurgensmeier JM (2002) Defective Bax activation in Hodgkin B-cell lines confers resistance to staurosporine-induced apoptosis. Cell Death Differ 9: 750–757
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60
Mallett S, Timmer A, Sauerbrei W, Altman DG (2010) Reporting of prognostic studies of tumour markers: a review of published articles in relation to REMARK guidelines. Br J Cancer 102: 173–180
McCormack PL, Keam SJ (2008) Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs 68: 487–506
Murukesh N, Dive C, Jayson GC (2010) Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer 102: 8–18
Ochiumi T, Tanaka S, Oka S, Hiyama T, Ito M, Kitadai Y, Haruma K, Chayama K (2004) Clinical significance of angiopoietin-2 expression at the deepest invasive tumor site of advanced colorectal carcinoma. Int J Oncol 24: 539–547
Ogawa M, Yamamoto H, Nagano H, Miyake Y, Sugita Y, Hata T, Kim BN, Ngan CY, Damdinsuren B, Ikenaga M, Ikeda M, Ohue M, Nakamori S, Sekimoto M, Sakon M, Matsuura N, Monden M (2004) Hepatic expression of ANG2 RNA in metastatic colorectal cancer. Hepatology 39: 528–539
Park JH, Park KJ, Kim YS, Sheen SS, Lee KS, Lee HN, Oh YJ, Hwang SC (2007) Serum angiopoietin-2 as a clinical marker for lung cancer. Chest 132: 200–206
Reinacher-Schick AC, Kubicka S, Freier W, Arnold D, Dietrich G, Geissler M, Hegewisch-Becker S, Graeven U, Schmoll H, Schmiegel W (2008) Activity of the combination of bevacizumab (Bev) with capecitabine/irinotecan (CapIri/Bev) or capecitabine/oxaliplatin (CapOx/Bev) in advanced colorectal cancer (ACRC): a randomized phase II study of the AIO Colorectal Study Group (AIO trial 0604). J Clin Oncol 26: 4030
Reiss Y, Knedla A, Tal AO, Schmidt MH, Jugold M, Kiessling F, Burger AM, Wolburg H, Deutsch U, Plate KH (2009) Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J Pathol 217: 571–580
Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118: 771–780
Sessa C, Guibal A, Del Conte G, Ruegg C (2008) Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol 5: 378–391
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK (2009) A ‘vascular normalization index’ as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69: 5296–5300
Thijssen VL, Brandwijk RJ, Dings RP, Griffioen AW (2004) Angiogenesis gene expression profiling in xenograft models to study cellular interactions. Exp Cell Res 299: 286–293
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147
Zhang L, Yang N, Park JW, Katsaros D, Fracchioli S, Cao G, O’Brien-Jenkins A, Randall TC, Rubin SC, Coukos G (2003) Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 63: 3403–3412
Acknowledgements
Dr U Fiedler (Heidelberg) is acknowledged for providing the human Ang-2 expression plasmid. VG and OC were funded by the Centre of Molecular Medicine Cologne (CMMC). Part of this work was funded by Mrs R Maassen (Erkelenz), the Moritz-Stiftung the Hilde-Kopp-Stiftung (Cologne) and the Marga and Walter Boll Stiftung (Kerpen).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Goede, V., Coutelle, O., Neuneier, J. et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer 103, 1407–1414 (2010). https://doi.org/10.1038/sj.bjc.6605925
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605925
Keywords
This article is cited by
-
Ang-2 is a potential molecular marker for lymphatic metastasis and better response to bevacizumab therapy in ovarian cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Angiopoietin-2-induced lymphatic endothelial cell migration drives lymphangiogenesis via the β1 integrin-RhoA-formin axis
Angiogenesis (2022)
-
Serum-based measurements of stromal activation through ADAM12 associate with poor prognosis in colorectal cancer
BMC Cancer (2022)
-
Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers
Journal of Biomedical Science (2021)
-
Angiopoietin-2 as a Prognostic Factor in Patients with Incurable Stage IV Colorectal Cancer
Journal of Gastrointestinal Cancer (2021)