Abstract
Background:
Thromboembolic events are a major complication in ovarian cancer patients. Tissue factor (TF) is frequently overexpressed in ovarian cancer tissue and correlates with intravascular thrombosis. TF binds to coagulation factor VII (fVII), changing it to its active form, fVIIa. This leads to activation of the extrinsic coagulation cascade. fVII is produced by the liver and believed to be supplied from blood plasma at the site of coagulation. However, we recently showed that ovarian cancer cells express fVII transcripts under normoxia and that this transcription is inducible under hypoxia. These findings led us to hypothesise that ovarian cancer cells are intrinsically associated with TF-fVIIa coagulation activity, which could result in thrombosis.
Methods:
In this study, we examined whether ectopically expressed fVII could cause thrombosis by means of immunohistochemistry, RT–PCR, western blotting and flow cytometry.
Results:
Ectopic fVII expression occurs frequently in ovarian cancers, particularly in clear cell carcinoma. We further showed that ovarian cancer cells express TF-fVIIa on the cell surface under normoxia and that this procoagulant activity is enhanced by hypoxic stimuli. Moreover, we showed that ovarian cancer cells secrete microparticles (MPs) with TF-fVIIa activity. Production of this procoagulant secretion is enhanced under hypoxia.
Conclusion:
These results raise the possibility that cancer cell-derived TF-fVIIa could cause thrombotic events in ovarian cancer patients.
Similar content being viewed by others
Main
Venous thromboembolism (VTE) is a common complication in cancer patients and a significant cause of morbidity and mortality (Chew et al, 2007; Varki, 2007). The risk of VTE is highest for cancers of the ovary, pancreas and liver (Iodice et al, 2008). In fact, ovarian cancer patients closely associate with intravascular thrombosis (Tateo et al, 2005; Satoh et al, 2007), and the development of VTE within 2 years is a strong predictor of death due to this cancer (Rodriguez et al, 2007). Activation of platelets and elevation of plasma tissue factor (TF) levels (Han et al, 2006; Tesselaar et al, 2007) are candidate determinants for the development of thrombosis in cancer patients.
Numerous studies have suggested that TF may have an important function in thrombosis in cancer patients. TF primarily presents on the cell surface as a transmembrane protein and functions as a cellular receptor for coagulation factor VII (fVII) circulating in the blood (Furie and Furie, 1988). By binding with TF, fVII is changed to its active form, fVIIa, and subsequently triggers the extrinsic coagulation cascade by activating factor X (Furie and Furie, 1988). Overexpression of TF correlates with a high incidence of VTE in pancreatic (Khorana et al, 2007) and ovarian (Uno et al, 2007) cancer patients. We have also reported that TF is constitutively expressed in many tumour cell lines (Koizume et al, 2006). Functionally active TF also circulates in the blood as a component of cell-derived microparticles (MPs) produced by platelets, cells with a monocyte/macrophage lineage (Lopez et al, 2005) and cancer cells (Amin et al, 2008). It is likely that this circulating TF may contribute to the development of thrombosis.
Hypoxia is a condition closely associated with cancer (Dewhirst et al, 2008), including ovarian cancer (Kim et al, 2006). The gene encoding TF is activated under hypoxia by a transcription factor, Egr-1, found in glioblastoma (Rong et al, 2005), melanoma, breast, lung (Amirkhosravi et al, 1998) and cervical cancer cells (Denko et al, 2000). To date, hypoxic induction of TF in ovarian cancer cells has not been reported.
In an earlier study, we showed that fVII, primarily synthesised in the liver, was constitutively produced in various non-hepatic cancer cells including ovarian cancer cells (Koizume et al, 2006). Furthermore, fVII transcription was inducible under hypoxic conditions by the hypoxia-inducible factor-2α (HIF-2α)-dependent pathway in ovarian cancer cells (Koizume et al, 2006). Thus, we surmised that fVII and TF induced by hypoxia in ovarian cancer cells, and not fVII derived from circulating blood, may be involved in thrombotic events in ovarian cancer patients.
The aim of this study was to examine the possibility that ovarian cancer tissue could intrinsically produce the TF-fVIIa complex independently of fVII supplied from blood plasma, and thus increase the risk of thrombosis. To this end, we investigated the expression of TF and fVII and the secretion of plasma membrane-derived MPs in ovarian cancer cells under both normoxia and hypoxia.
Materials and methods
Cell lines and cell culture
Ovarian cancer cell lines including four clear cell carcinoma (OVSAYO, OVISE, OVMANA, and OVTOKO); two serous adenocarcinoma (OVSAHO and OVKATE); one mucinous adenocarcinoma (MCAS); one endometrioid adenocarcinoma (TOV112D) and three undifferentiated carcinoma (KURAMOCHI, NIH:OVCAR-3, and TYK-nu) cell lines were used in this study. TOV112D and NIH:OVCAR-3 were obtained from the American Type Culture Collection (Manassas, VA, USA). Other cell lines were described earlier (Koizume et al, 2006). All cell lines were cultured in RPMI-1640 medium supplemented with 10% foetal bovine serum (Moregate, Brisbane, Australia) at 37°C in a humidified atmosphere under 5% CO2.
Real-time RT–PCR analysis of FVII and TF gene expression
Cancer cells were harvested at the exponential growth phase. Cells (2 × 106 cells) were seeded on to a 100 mm-diameter dish and cultured for 16 h. Cells were further cultured under normoxia or hypoxia (1% O2) as described earlier (Koizume et al, 2006). Total RNA isolated from cancer cells was subjected to real-time RT–PCR analysis as described earlier (Koizume et al, 2006). The PCR primers used for TF expression were 5′-TAACCGGAAGAGTACAGACAGC-3′ and 5′-CACTCCTGCCTTTCTACACTTG-3′. The hybridisation probes used were 5′-ATCATTGGAGCTGTGGTATTTGTGG-FITC-3′ and 5′-LCRed640-CATCATCCTTGTCATCATCCTGGC-3′. Expression of porphobilinogen deaminase was analysed as described earlier (Koizume et al, 2006) to normalise data.
Isolation of MPs
To isolate cell-derived MPs, conditioned media were filtered through a 5 μm-pore membrane, then ultracentrifuged at 100 000 g for 90 min at 10°C according to a published procedure (Davila et al, 2008). To isolate MPs secreted under serum-free condition, cultured cells were washed twice with 5 ml of PBS, then cultured in serum-free media for 3 h. Media were then replaced with new serum-free media and further cultured under normoxia or hypoxia for 24 h. Obtained conditioned media were processed as described above.
fXa generation assay
The activated factor X (fXa) generation assay was performed as described earlier (Koizume et al, 2006) with a slight modification of seeded cell numbers (1 × 105 cells per well of a 24-well plate). Briefly, conditioned media were collected and filtered through a 5 μm-pore filter (Millex-SV, Millipore, Billerica, MA, USA) to remove cell debris. Six microlitres of 250 mM CaCl2 (final concentration was 5 mM) and 175 nM fX were added to 294 μl of the filtered conditioned medium. After incubation at 37°C for 4 and 2 h for cells and conditioned media, respectively, a 20 μl aliquot was removed and used for the analysis. In addition, MPs were resuspended in reaction buffer and subjected to fXa generation analysis.
The tilt tube plasma clotting assay
Tilt tube plasma clotting assay was performed as described earlier (Rong et al, 2005) with slight modifications. The filtered conditioned medium (200 μl) was mixed with 200 μl of citrated normal human plasma (George King Bio-Medical, Inc., Overland Park, KS, USA), and then 200 μl of 25 mM CaCl2 was added to the tube to initiate the semisolid gel formation. The times required for clot formation were recorded to evaluate procoagulant activity.
Flow cytometry
MPs of 15 μl were diluted with 85 μl of binding buffer (0.01 M Hepes [pH 7.4], 140 mM NaCl and 2.5 mM CaCl2), and then each sample was incubated with 5 μl of FITC annexin V (BD-Pharmingen, San Jose, CA, USA) and/or 20 μl of PE-conjugated monoclonal anti-TF antibody (BD-Pharmingen) for 15 min at room temperature. To detect fVII on the MPs, diluted MPs were incubated with 10 μl of monoclonal anti-fVII antibody (F8146, Sigma, Saint Louis, MO, USA) labelled with FITC using DyLight Antibody Labeling Kits (Thermo Scientific, Rockford, IL, USA). We used unlabelled samples, an isotype-matched mouse IgG-PE or mouse IgG-FITC (BD-Pharmingen) as controls. MPs were analysed by size, using 1 μm calibration microspheres (Molecular Probes Inc., Eugene, OR, USA), and fluorescence using a Coulter Epics Altra flow cytometer (Beckman Coulter Inc., San Diego, CA, USA).
Western blotting
Immunoblotting was performed using whole-cell lysates of cancer cells. The antibodies used were the anti-TF monoclonal antibody (TF9 10H10), the anti-Egr-1 (sc-110, Santa Cruz Biotechnology Inc., CA, USA), the anti-Sp-1 (sc-59, Santa Cruz) and the anti-β-actin (A5441, Sigma) antibodies. Detection of antibodies was achieved using enhanced chemiluminescence reagents (Thermo Scientific).
Immunohistochemistry
The institutional review board at Yokohama City University approved this study. Surgically removed ovarian carcinomas were obtained from the Department of Obstetrics and Gynecology, Yokohama City University Hospital. Routinely processed formalin-fixed paraffin-embedded specimens were sectioned and stained with antibodies for TF (TF CPT at 7.6 μg ml−1) and fVII (fVII R0882 at 10 μg ml−1). Immunoreactivity was visualised by the peroxidase-labelled amino acid polymer method with Histofine simple stain MAX-PO(R) (Nichirei Co., Tokyo, Japan) and by the avidin–biotin–peroxidase complex method (LSAB+, DakoCytomation Co., Tokyo, Japan) according to the manufacturer's instructions. Sections were counterstained with haematoxylin. Data were obtained using the modified German immunoreactive score (Krajewska et al, 1996). Briefly, immunostaining intensity was rated using four ranks ranging from zero (none) to three (intense). The numbers of immunoreactive cancer cells were also estimated in four grades as zero (none), one (1–10% per cancer cells), two (10–50%) and three (50%<). Two representative areas were evaluated for each case, and the sum of the scores was used for the score of that case. Two independent examiners scored each case and the average value was used for analyses.
Results
Ectopic expression of fVII in human epithelial ovarian carcinoma
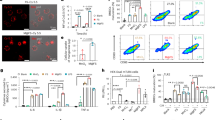
We first examined the expression of TF and fVII in ovarian cancer tissues using 20 surgically removed specimens. The specimens consisted of five serous, three mucinous and three endometrioid adenocarcinomas, as well as seven clear cell carcinomas and one normal premenopausal ovary with normal ovarian surface epithelium (OSE) (Figure 1). Immunohistochemical analysis revealed a strong TF expression in mucinous and endometrioid adenocarcinomas, in addition to clear cell carcinomas as reported earlier (Uno et al, 2007) (Figure 1A and B). TF was less strongly expressed in serous adenocarcinomas. TF was not detected in OSE (Figure 1A). Immunohistochemistry also revealed that fVII was expressed in all ovarian cancer specimens examined, particularly in clear cell carcinomas (Figure 1A and B).
Immunohistochemical analysis of TF and fVII expressions in ovarian cancer tissues. (A) Expression level of TF and fVII in ovarian cancer and normal ovarian surface epithelium (OSE) of a pre-menopausal ovary. Ovarian cancer tissues from serous, mucinous, endometrioid adenocarcinomas, clear cell carcinoma and OSE were stained with TF or fVII antibody. Expression levels were rated using the modified German immunoreactive score. *P=0.002, **P=0.039 (Student's t-test). (B) A representative example of immunohistochemical detection of TF and fVII in an ovarian clear cell carcinoma tissue that was scored as 11.5 and 11.0 for TF and fVII, respectively.
Hypoxia induction of fVII and TF transcription in ovarian cancer cells of various histological types
Under normoxia, we showed earlier that OVSAYO, OVISE, OVSAHO and OVTAKE cells weakly expressed fVII mRNA and that KURAMOCHI strongly expressed fVII mRNA. In the past, hypoxic FVII gene induction has been examined in detail only for OVSAYO cells (Koizume et al, 2006). Thus, in this study, we examined whether the FVII gene was inducible using hypoxia in additional ovarian cancer cell lines. We first used 500 μ M CoCl2 to mimic hypoxic conditions, as this approach has been shown to stimulate higher levels of HIFs in cancer cells, as compared with real hypoxic (1% O2) conditions (Koizume et al, 2008). Real-time RT–PCR analysis revealed that fVII transcription was clearly inducible by CoCl2 in several cell lines (Figure 2A). Constitutively expressed TF mRNA in ovarian cancer cells (Koizume et al, 2006) was also found to be upregulated in some cells after CoCl2 treatment (Figure 2A).
Real-time RT–PCR analysis of TF and fVII expressions in ovarian cancer cells. (A) Relative fVII and TF mRNA levels in ovarian cancer cells stimulated with 500 μ M CoCl2 for 4 h. fVII transcription in OVSAYO and OVSAHO cells was reported earlier (Koizume et al, 2006) but was re-examined for comparison. *P<0.05, ND; not detected. (B) Relative increase of fVII and TF transcripts in OVSAYO and OVISE cells cultured under 1% O2 for 24 h compared with those cultured under normoxia (*P<0.001, **P=0.023, ***P=0.016, ****P=0.003). Columns for fVII and TF mRNA levels, mean (n=3); Bars, s.d. Data were statistically analysed using Student's t-test. (C) Western blotting analysis of TF levels in ovarian cancer cells. N and H are indicative of normoxia and hypoxia, respectively. (D) Western blotting analysis of Egr-1 and Sp1 expressions in ovarian cancer cells cultured under normoxia, hypoxia (1% O2 for 16 h) or hypoxia mimic (500 μ M CoCl2 for 4 h) conditions. (E) Induction of procoagulant activity on the cell surface of ovarian cancer cells under CoCl2 or hypoxic conditions. Ovarian cancer cells were cultured under 500 μ M CoCl2 for 11 h or under 1% O2 for 16 h, and then subjected to fXa generation assay. Columns for clotting time, mean (n=3); Bars, s.d. *P=0.001, **P=0.002, ***P<0.001, ****P=0.012.
We next carried out a study under real hypoxic conditions (1% O2 for 24 h) for fVII and TF transcriptional activation in clear cell carcinoma cell lines OVSAYO and OVISE. Immunohistochemistry and RT–PCR analyses revealed that this histological subtype could highly express fVII and TF transcripts. Quantitative RT–PCR analysis revealed that real hypoxia also induced fVII and TF transcriptions (Figure 2B). TF mRNA levels were quite high under normoxia and hypoxia, compared with those of ectopically expressed fVII (Koizume et al, 2006), enabling us to identify TF protein by western blotting (Figure 2C).
Transcription factor Egr-1 is known to be upregulated and has a dominant function over Sp1 for TF gene induction under hypoxia (Yan et al, 1998; Rong et al, 2006). As a consequence, we additionally tested the expression of Egr-1 in ovarian cancer cell lines. Western blotting revealed that Egr-1 expression was enhanced under CoCl2 or 1% O2 conditions. In contrast, expression of Sp1, which is responsible for basal TF transcription, was unchanged in these cell lines (Figure 2D).
Hypoxia-induced procoagulant activity in ovarian cancer cells
The TF-fVIIa complex cleaves fX to generate fXa, further activating the extrinsic coagulation cascade. To evaluate whether TF and fVII expressions in ovarian cancer cells caused the procoagulant reaction, we performed the fXa generation assay. OVSAYO and OVISE cells were cultured under normoxic, mimicked hypoxic (500 μ M CoCl2) and real hypoxic (1% O2) conditions. They were then tested for coagulation activity. We found that the fXa-generating activity of OVSAYO cells (10.6 pM per min) and OVISE cells (13.7 pM per min) was significantly higher than that of the cell-free control experiment (Figure 2E), suggesting that OVSAYO and OVISE cells expressed procoagulant activity on their surface under normoxia. As expected, both real hypoxic and CoCl2-induced conditions enhanced procoagulant activity on OVSAYO and OVISE cells (Figure 2E). Further, the addition of anti-TF (5G9) antibody markedly reduced the procoagulant activity, although normal IgG failed to inhibit it (Figure 2E). This suggested that the TF-fVIIa complex was responsible for the observed procoagulant activity.
TF-fVIIa activity in ovarian cancer cells under normoxia and hypoxia
Increased plasma TF levels have been reported to correlate with thrombosis in several cancer patients. In this context, we showed that ovarian cancer cells could intrinsically express TF-fVIIa activity on their surface. Thus, we next investigated whether ovarian cancer cells could secrete the TF-fVIIa complex without fVII derived from blood plasma. OVSAYO and OVISE cells were cultured under normoxia or 1% O2, and conditioned media were subsequently collected. These conditioned media were then mixed with citrated human normal plasma supplemented with CaCl2, and gently rocked for the tilt tube assay. The clotting time achieved using conditioned media, prepared from cells cultured under normoxia, was markedly reduced as compared with experiments using cell-free medium (Figure 3). As expected, experiments with conditioned media, prepared from cells cultured under hypoxia, revealed that clotting times were further shortened (Figure 3). The coagulation reactions were completely blocked by 5G9 antibody treatment (data not shown). These results suggested that ovarian cancer cells could secrete functional TF.
Secretion of procoagulant activity by ovarian cancer cells. Ovarian cancer cells were cultured under normoxia or 1% O2 for 24 h, and then conditioned media were subjected to the plasma clotting assay using normal or fVII-deficient human plasma. FVII-containing cells and empty cells were indicative of stably fVII cDNA-transfected cells and stably transfected empty vector cells, respectively. Columns for clotting time, mean (n=3); Bars, s.d. *P=0.009, **P=0.003, ***P=0.002, ****P=0.0001.
We further performed the same clotting assay using fVII-deficient plasma. As expected, clotting times were generally longer than those recorded in experiments using normal fVII-containing plasma (Figure 3). However, plasma clotting was considerably enhanced under hypoxia in the absence of fVII (Figure 3). As expected, positive control experiments using fVII-overexpressing OVSAYO cells (Koizume et al, 2006) showed further reduced clotting times, in comparison with experiments involving empty vector-transfected cells (Figure 3). This suggested that ectopically synthesised fVII is secreted as a functional procoagulant in ovarian cancer cells.
Secretion of MPs with TF-fVIIa activity by ovarian cancer cells
TF has been shown to circulate in the blood as a component of cell-derived MPs released as fragments of plasma membrane. Secretion of TF-positive MPs has been found to be increased and associated with the activation of the haemostatic system in several cancer patients (Hron et al, 2007; Davila et al, 2008). Following up on this finding, we next investigated whether TF-fVIIa secreted from ovarian cancer cells was associated with the activity of MPs. Conditioned media from ovarian cancer cell culture under 1% O2 were ultracentrifuged. Coagulation activity was then assessed for both supernatant and precipitate fractions. The fXa generation assay revealed that TF-fVIIa activity was predominantly enhanced in the precipitate that included MPs (Figure 4A). As expected, procoagulant activity was blocked by anti-human TF antibody treatment (Figure 4A).
Secretion of MPs with TF-fVIIa activity by ovarian cancer cells. (A) fXa generation activity of the supernatant and precipitate fraction of conditioned media prepared from OVSAYO and OVISE cells cultured under 1% O2. Procoagulant activity predominantly presented in the precipitate fraction and this activity was completely blocked by anti-TF (5G9) antibody treatment. *P<0.001, **P=0.001. (B) Flow cytometry analysis of precipitate fraction immunostained with PE-coupled anti-TF antibody and/or FITC-coupled annexin V showed that precipitate fraction prepared from conditioned media of OVSAYO cells was annexin-V positive. (C) The extracellular procoagulants analysed by flow cytometry. The grey peak corresponds to the 1-μm calibration microspheres. The white peak corresponds to the precipitate fraction prepared from conditioned media of OVSAYO cells. (D) Flow cytometry analysis of the precipitate fractions prepared from serum-free conditioned media of OVSAYO cells cultured under normoxia or 1% O2 for 24 h. (Da) The MP fraction prepared was TF positive. Background fluorescence was set with a fluorescent mouse IgG control. (Db) Dot plot of MP fraction immunostained with PE-coupled anti-TF antibody and FITC-coupled annexin V. Percentages shown in the upper right segment of the figure denote the relative amount of TF-positive MPs. (E) Flow cytometry analysis of the precipitate fractions prepared from serum-free conditioned media of OVSAYO cells cultured under normoxia or 1% O2 for 24 h. (Ea) The MP fraction prepared was fVII positive. Background fluorescence was set with a fluorescent mouse IgG control. (Eb) Dot plot of MP fraction immunostained with PE-coupled anti-TF antibody and FITC-coupled anti-fVII antibody. Percentages in the upper right segment of the figure denote amounts of fVII-positive fractions relative to total MPs.
We performed flow cytometry to further examine whether the activity of TF-fVIIa secreted by ovarian cancer cells was associated with MPs. The analysis revealed that most of the particles (∼90%) present in the precipitate from the serum-free conditioned media bind to annexin V. They were smaller than 1 μm in size (Figure 4B and C), thus satisfying the criteria for being MPs. MPs were also found to be TF positive by FACS analysis (Figure 4Da). The number of TF-positive MPs secreted into serum-free conditioned media from OVSAYO cells, maintained under 1% O2, increased by ∼1.3 times compared with total MPs, including microvesicles not associated with TF (Figure 4Db). Flow cytometry with anti-human fVII antibody showed that the TF-positive MP fraction prepared from serum-free culture media was also fVII positive (Figure 4Ea and Eb), eliminating the possibility that pre-existing trace fVII in bovine serum was responsible for binding TF-positive MPs. The number of fVII-positive MPs was increased under hypoxia relative to the number of TF-positive MPs (Figure 4E). These results suggested that ovarian cancer cells secreted TF-fVIIa-positive MPs.
Discussion
In this study, we showed that not only TF but also fVII proteins were localised to cancer cells in surgically removed ovarian cancer tissue specimens. This was especially true in the case of clear cell carcinomas. Unlike TF expressions, the fVII expression evaluated by immunohistochemistry was generally weak, except for clear cell carcinomas, and was comparable with that of a normal ovarian tissue. fVII that came from blood plasma perhaps cross-reacts in histochemical analysis. Furthermore, we found that hypoxic conditions induced the expression of the FVII gene, together with the TF gene, in various types of ovarian cancer cells. We showed that slight TF-fVIIa activity on the surface of ovarian cancer cells under normoxia was enhanced under hypoxic conditions. We further showed that TF-fVIIa activity was not only localised on the cancer cell surface, but was also secreted into culture media as a component of MPs. Hypoxia was found to accelerate this process.
The TF-fVIIa complex on the ovarian cancer cell surface may trigger the extrinsic coagulation cascade within or around ovarian cancer tissue. TF-fVIIa on the cell surface is also known to transduce various kinds of signals within cells. Both the products of the coagulation cascade and transduced signals in cells have important functions in cancer biology, such as cell invasion and migration, angiogenesis and cell survival through intracellular signalling mechanisms (Ruf, 2007). We showed earlier that ectopic synthesis of fVII in OVSAYO cells, followed by TF-fVIIa formation on the cell surface, markedly enhanced cell motility and invasion (Koizume et al, 2006). Ovarian cancer is highly metastatic and tumour cell migration from the primary site to the peritoneal cavity is the critical process of ovarian cancer spread. Given that cells in primary ovarian cancer tissue and ovarian cancer cells in ascites undergo hypoxic stress (Kim et al, 2006), self-production of TF-fVIIa on the cell surface may contribute to metastasis of ovarian cancer.
In addition to cell surface localisation, TF could circulate in the blood even under normal conditions. TF is found in the blood as an alternatively spliced soluble form without the transmembrane domain or as a component of cell-derived MPs. Most of the TF-derived procoagulant activity is associated with MPs (Yu and Rak, 2004). TF-positive MPs in different types of cancer patients are known to increase in response to various cellular stresses (Amin et al, 2008). It is likely that cancer cell-derived MPs contribute to hypercoagulability in those patients, as various types of cancer cells are known to secrete TF-positive MPs (Amin et al, 2008). In these cases, procoagulant activity of TF-positive MPs was considered to depend on blood-derived fVII. In this study, we have provided novel evidence that MPs containing active TF-fVIIa complex are secreted directly from ovarian cancer cells, and that this phenomenon is enhanced under hypoxia. Given that ovarian cancer patients have a high incidence of VTE at distant sites from neoplastic tissue (Tateo et al, 2005; Satoh et al, 2007), cancer cell-derived TF-fVIIa-positive MPs may have a function in intravascular thrombosis in such patients.
Elevated incidences of VTE have been reported to be associated with tumour size in gliomas (Marras et al, 2000). Indeed, increase in tumour volume is associated with progressive development of hypoxia in many cancer tissues. Thus, it is probable that hypoxic stresses that are promoted as ovarian cancer grows, especially in the peritoneal cavity, accentuate the secretion of TF-fVIIa-positive MPs, leading to the development of VTE. Future investigations into the relationship between the ectopic expression of fVII in tumour tissue; plasma concentrations of TF or TF-fVIIa-positive MPs; and the incidence of VTE in ovarian cancer patients will lead to a greater understanding of the mechanisms of thrombosis in cancer patients.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amin C, Mackman N, Key NS (2008) Microparticles and cancer. Pathophysiol Haemost Thromb 36: 177–183
Amirkhosravi A, Meyer T, Warnes G, Amaya M, Malik Z, Biggerstaff JP, Siddiqui FA, Sherman P, Francis JL (1998) Pentoxifylline inhibits hypoxia-induced upregulation of tumor cell tissue factor and vascular endothelial growth factor. Thromb Haemost 80: 598–602
Chew HK, Wun T, Harvey DJ, Zhou H, White RH (2007) Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 25: 70–76
Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL (2008) Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 6: 1517–1524
Denko N, Schindler C, Koong A, Laderoute K, Green C, Giaccia A (2000) Epigenetic regulation of gene expression in cervical cancer cells by the tumor microenvironment. Clin Cancer Res 6: 480–487
Dewhirst MW, Cao Y, Moeller B (2008) Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8: 425–437
Furie B, Furie BC (1988) The molecular basis of blood coagulation. Cell 53: 505–518
Han LY, Landen Jr CN, Kamat AA, Lopez A, Bender DP, Mueller P, Schmandt R, Gershenson DM, Sood AK (2006) Preoperative serum tissue factor levels are an independent prognostic factor in patients with ovarian carcinoma. J Clin Oncol 24: 755–761
Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A (2007) Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost 97: 119–123
Iodice S, Gandini S, Lohr M, Lowenfels AB, Maisonneuve P (2008) Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost 6: 781–788
Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB (2007) Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 13: 2870–2875
Kim KS, Sengupta S, Berk M, Kwak YG, Escobar PF, Belinson J, Mok SC, Xu Y (2006) Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res 66: 7983–7990
Koizume S, Jin MS, Miyagi E, Hirahara F, Nakamura Y, Piao JH, Asai A, Yoshida A, Tsuchiya E, Ruf W, Miyagi Y (2006) Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res 66: 9453–9460
Koizume S, Yokota N, Miyagi E, Hirahara F, Tsuchiya E, Miyagi Y (2008) Heterogeneity in binding and gene-expression regulation by HIF-2alpha. Biochem Biophys Res Commun 371: 251–255
Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC (1996) Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol 148: 1567–1576
Lopez JA, del Conde I, Shrimpton CN (2005) Receptors, rafts, and microvesicles in thrombosis and inflammation. J Thromb Haemost 3: 1737–1744
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 89: 640–664
Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH (2007) Venous thromboembolism in ovarian cancer. Gynecol Oncol 105: 784–790
Rong Y, Hu F, Huang R, Mackman N, Horowitz JM, Jensen RL, Durden DL, Van Meir EG, Brat DJ (2006) Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1-independent mechanisms. Cancer Res 66: 7067–7074
Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ (2005) PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 65: 1406–1413
Ruf W (2007) Tissue factor and PAR signaling in tumor progression. Thromb Res 120 (Suppl 2): S7–S12
Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, Minami R, Matsumoto K, Tanaka YO, Tsunoda H, Homma S, Yoshikawa H (2007) High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer 97: 1053–1057
Tateo S, Mereu L, Salamano S, Klersy C, Barone M, Spyropoulos AC, Piovella F (2005) Ovarian cancer and venous thromboembolic risk. Gynecol Oncol 99: 119–125
Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S (2007) Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost 5: 520–527
Uno K, Homma S, Satoh T, Nakanishi K, Abe D, Matsumoto K, Oki A, Tsunoda H, Yamaguchi I, Nagasawa T, Yoshikawa H, Aonuma K (2007) Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer 96: 290–295
Varki A (2007) Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood 110: 1723–1729
Yan SF, Zou YS, Gao Y, Zhai C, Mackman N, Lee SL, Milbrandt J, Pinsky D, Kisiel W, Stern D (1998) Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA 95: 8298–8303
Yu JL, Rak JW (2004) Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost 2: 2065–2067
Acknowledgements
This study was partly supported by a Grant-in-Aid for the Encouragement of Basic Science and Technology from the Science and Technology Office of the Kanagawa Prefectural Government, and by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yokota, N., Koizume, S., Miyagi, E. et al. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. Br J Cancer 101, 2023–2029 (2009). https://doi.org/10.1038/sj.bjc.6605406
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605406
Keywords
This article is cited by
-
Validation of tissue factor pathway inhibitor 2 as a specific biomarker for preoperative prediction of clear cell carcinoma of the ovary
International Journal of Clinical Oncology (2021)
-
Lipid starvation and hypoxia synergistically activate ICAM1 and multiple genes in an Sp1-dependent manner to promote the growth of ovarian cancer
Molecular Cancer (2015)
-
MicroRNA-21 is a candidate driver gene for 17q23-25 amplification in ovarian clear cell carcinoma
BMC Cancer (2014)
-
Risk of ischemic stroke in patients with ovarian cancer: a nationwide population-based study
BMC Medicine (2014)
-
Expression of coagulation factors and their receptors in tumor tissue and coagulation factor upregulation in peripheral blood of patients with cerebral carcinoma metastases
Journal of Cancer Research and Clinical Oncology (2012)