Abstract
Increased retinoic acid receptor β (RARβ2) gene expression is a hallmark of cancer cell responsiveness to retinoid anticancer effects. Moreover, low basal or induced RARβ2 expression is a common feature of many human cancers, suggesting that RARβ2 may act as a tumour suppressor gene in the absence of supplemented retinoid. We have previously shown that low RARβ2 expression is a feature of advanced neuroblastoma. Here, we demonstrate that the ABC domain of the RARβ2 protein alone was sufficient for the growth inhibitory effects of RARβ2 on neuroblastoma cells. ATP7A, the copper efflux pump, is a retinoid-responsive gene, was upregulated by ectopic overexpression of RARβ2. The ectopic overexpression of the RARβ2 ABC domain was sufficient to induce ATP7A expression, whereas, RARβ2 siRNA blocked the induction of ATP7A expression in retinoid-treated neuroblastoma cells. Forced downregulation of ATP7A reduced copper efflux and increased viability of retinoid-treated neuroblastoma cells. Copper supplementation enhanced cell growth and reduced retinoid-responsiveness, whereas copper chelation reduced the viability and proliferative capacity. Taken together, our data demonstrates ATP7A expression is regulated by retinoic acid receptor β and it has effects on intracellular copper levels, revealing a link between the anticancer action of retinoids and copper metabolism.
Similar content being viewed by others
Main
Retinoic acid receptors are members of the nuclear receptor super family, which act as ligand-regulated trans-acting transcription factors that bind to cis-acting DNA regulatory elements in the promoter regions of target genes. Three RAR subtypes (RARα, RARβ, and RARγ) originating from three distinct genes have been identified (Mangelsdorf et al, 1990; Gronemeyer et al, 2004). RARs belong to the steroid/thyroid hormone receptor super-family, which share a common domain structure, denoted A-F. The C region contains the DNA-binding domain, which is most conserved among the different members of this family and consists of two zinc fingers. The hormone-binding domain is located in the E region and contains, besides the binding domain, a dimerisation domain and a hormone-dependent transactivation function (AF-2). The N-terminal part of the receptor (AB) also contains an autonomous region involved in transactivation (AF-1), which functions independently from the ligand, when coupled with a heterologous DNA-binding domain. As the NH2-terminus is not conserved among different receptors, they each may achieve this function by distinct means (Evans, 1988; Leid et al, 1992).
Of particular interest is the RARβ2 subtype, which regulates essential pathways associated with the anti-tumour effects of retinoids in epithelial cells. The RARβ2 gene promoter contains a retinoic acid response element (RARE) to which the liganded RARα/RXRα heterodimer binds to induce RARβ2 expression (Alvarez et al, 2007). The expression of RARβ2 is frequently reduced in many neoplastic tissues when compared with their normal cellular counterparts, including non-small cell lung cancer, squamous cell carcinomas of the head and neck, breast cancer and neuroblastoma (Cheung et al, 1996; Castillo et al, 1997; Picard et al, 1999). The reactivation of silenced RARβ2 upon induction of RARα expression and concomitant retinoic acid treatment was associated with a clinical response of oral leukoplakia (Lotan et al, 1995). In a clinical study where renal carcinoma patients were treated with 13-cis-retinoic acid and interferon-α, the levels of RARβ2 increased in tumours that clinically responded to therapy (Berg et al, 1999).
Copper plays an essential role in human physiology and is indispensable for normal growth and development. Enzymes that are involved in connective tissue formation, neurotransmitter biosynthesis, iron transport and other essential physiological processes require copper as a cofactor to mediate their reactions. The transport and cellular metabolism of copper depends on a series of membrane proteins and smaller soluble peptides that comprise a functionally integrated system for maintaining cellular copper homeostasis. Two membrane-bound copper-transporting ATPase enzymes, ATP7A and ATP7B, can catalyse an ATP-dependent transfer of copper to intracellular compartments or expel copper from the cell (Harris, 2000; El Meskini et al, 2005), respectively. A truncated splice variant of ATP7A, termed NML45 (Nuclear Menkes Like) has also been described that encodes a theoretical 11.2-kDa protein with one copper-binding site, and may function as a nuclear copper chaperone (Reddy et al, 2000).
We have previously identified the copper metabolism gene ATP7A as a retinoid-regulated gene in neuroblastoma cell lines (Liu et al, 2005). In non-neuronal cells, ATP7A is responsive to levels of intracellular copper, and is predominantly localised to the trans-Golgi network when cytosolic copper levels are normal or low. In the presence of excess copper, ATP7A functions in copper excretion and a distinct pool of ATP7A is relocated to the plasma membrane through cytoplasmic vesicles. It is rapidly recycled, returning to the trans-Golgi network once cytoplasmic copper levels are normal (La Fontaine et al, 1999; Lutsenko and Petris, 2003). In neurons ATP7A is developmentally regulated, being initially expressed in cell bodies of developing neurons, with later expression shifting to the extending axons and peaking just prior to synaptogenesis (El Meskini et al, 2005). ATP7A has an important function in neurodevelopment, and is involved in axonal targeting, synaptogenesis and maturation of olfactory sensory neurons (El Meskini et al, 2007).
In this study, we investigated the mechanisms of RARβ2 in mediating ligand-dependent and ligand-independent growth inhibition functions by using in vitro models. Our results show a previously unrecognised relationship between the RARβ2 and the anticancer effects of retinoids with ATP7A and intracellular copper levels.
Materials and methods
Cell culture
The human neuroblastoma BE(2)-C and SH-SY5Y cell lines were generously supplied by Dr J Biedler (Memorial Sloan-Kettering Cancer Centre, NY, New York, USA). The human lung cancer H441 and SK-MES-1, human breast cancer MDA-MB-468, MDA-MB-231 and MCF7, and skin immortal keratinocyte HEK001 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The HEK001 was cultured in a specific keratinocyte serum-free media as specified by the ATCC. All other cells were cultured in Dulbecco's modified Eagle's medium supplemented with L-glutamine and 10% fetal calf serum. All-trans-retinoic acid (aRA) was purchased from Sigma (NSW, Australia), was solubilised in ethanol, stored at −70°C, and used within 2 weeks of solubilisation. Triethylenetetramine (TETA), a selective copper chelator, was also purchased from Sigma.
Semi-quantitative competitive reverse transcriptase–polymerase chain reaction
The competitive RT–PCR techniques have been described earlier (Norris et al, 1997; Cheung et al, 2003), which involved determining a ratio between the level of expression of a target gene and that of the housekeeping gene β2-microglobulin (β2M) in total RNA samples. Primers used for RT–PCR assays are as follows: human ATP7A forward: 5′-GGAATTCCCATAGCTGCTGG-3′; human ATP7A reverse: 5′GTAAGTTGGTTTCCTGTAAA-3′. This primer pair generated a 140-bp product extending from 4233 to 4373 bp of published ATP7A sequence. NLM45 forward: 5′-CTGAGCATCAGAAAGAGACCA-3′; NML45 reverse: 5′-ATCTGCTGCTCAATGGTCCA-3′. This primer pair generated a 146-bp product extending from 122 to 146 bp of published NML45 sequence. Human RARβ2 forward: 5′-CTACACTGCGAGTCCGTCTT-3′. Human RARβ2 reverse: 5′-CAGAGCTGGTGCTCTGTGTT-3′. This primer pair generated a 131-bp product extending from 375 to 506 bp of published RARβ2 sequence.
Quantitative real-time PCR
cDNA for real-time PCR was synthesized from total RNA using a Superscript III synthesis kit and Oligo(dT)20 primer (Invitrogen, Vic, Australia), according to manufacturer instructions. The cDNA (40 ng) was amplified in 25 μl reactions using a Power SYBR Green PCR master mix (Applied Biosystems, Melbourne, VIC, Australia) and 100 ng each of forward and reverse primers on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The cycle threshold (Ct), fixed in the exponential phase of the PCR, was used to determine gene expression relative to a calibrator. For each sample, the Ct value of the target gene was normalised to the endogenous control gene β2M. Relative gene expression was calculated with the method (Livak and Schmittgen, 2001). Real-time PCR primer sequences are listed as follows: Human ATP7A forward: 5′-TGGCAAGGCAGAAGTAAGGTATAA-3′; Human ATP7A Reverse: 5′-ACGTCATTCCCCTCACAACAAG-3′; Human RARβ2 forward: 5′-TGAAAATCACAGATCTCCGTAGCA-3′; Human RARβ2 reverse: 5′-CCAGGAATTTCCATTTTCAAGGT-3′; Human β2M forward: 5′ACTGGTCTTTCTATCTCTTGTACTACACTGA-3′; Human β2M reverse: 5′ TGATGCTGCTTACATGTCTCGAT-3′.
Plasmid construction
The full-length human cDNA for RARβ2 (kindly provided by Professor P Chambon, INSERM, Strasbourg, France) was cloned into the PvuII multi-cloning site of the pMEP4 episomal Epstein–Barr virus-based expression vector (kindly provided by Dr M Tykocinski, Case Western University, Cleveland, OH, USA) (Cheung et al, 1996). We designed mutant RARβ2 cDNAs, which were deleted for either the ligand-independent AF-1 transactivating domain (A-B) and the DNA-binding domain (C) – ΔDEF, or the ligand-binding domain (E-F) – ΔABC and ΔAB (Figure 1B). We used a PCR-based strategy to amplify separate regions of the RARβ cDNA by using pREP/RARβ plasmid DNA as template (Cheung et al, 1996). The mutant RARβ cDNAs were subcloned into the multi-cloning site (Hind III and Cla I) of a pTet-splice Myc/His vector (Invitrogen). Plasmid pTet-tTAK (Invitrogen) was stably transfected into BE(2)-C cells to establish stable BE(2)-C pTet-tTAK clones. The pTet-splice vectors which contained the RARβ mutants were transiently transfected into BE(2)-C (pTet-tTAK clones) and BrdU incorporation was performed in medium without tetracycline.
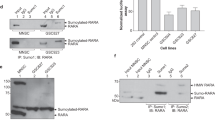
Tumour-suppressive activity of RARβ2 in neuroblastoma tumour cells. (A) BE(2)-C and SH-SY5Y cells were transfected with control siRNA or RARβ2-specific siRNA, followed by treatment with 10 μ M aRA for 48 h and incubation with BrdU for the last 2 h. In the upper panel, BrdU incorporation was measured as OD units of absorbance. In the lower panel, RT–PCR experiments show the knock-down of RARβ2 mRNA expression level by RARβ2-specific siRNA. (B) Schematic representations of RARβ2 full-length and deletion mutant constructs used in this study. In the right panel, expression of RARβ2 full length and deletion mutants in BE(2)-C pTet-tTAK cells were analysed by western blot by using anti-Myc-tag antibody. The lysates of the cells transiently transfected with empty vector (EV, lane 1), RARβ2 full-length (lane 2, 50 KDa), ΔDEF (lane 3, 16 kDa), ΔABC (lane 4, 34 kDa) and ΔAB (lane 5, 41 kDa) constructs were used in this study. (C) Proliferation of BE-tet-75 cells transiently transfected with RARβ2 full-length and deletion mutant constructs in the absence of added retinoid. *P<0.05 and **P<0.01 indicate a statistically significant difference compared with untreated control (Figure 1A) or empty vector control (Figure 1C). Error bars indicate s.e.m.
The oligonucleotides used for the PCR assay of RARβ deletion mutants were as follows: Forward primer for ΔDEF: 5′-AGTCGAGGTATCGATGTTTGACTGTATGGATGT-3′; Reverse primer for ΔDEF: 5′-AGCAGAAAGCTTGGACATTCCCACTTCAAAGC-3′; Forward primer for ΔAB: 5′-AGTCGAGGTATCGATGGCTTCGTCTGCCA-3′; Forward primer for ΔABC: 5′-AGTCGAGGTATCGATGAAAGAATCTGTCAGGAA-3′; Reverse primer for both ΔAB and ΔABC: 5′-AGCAGAAAGCTTAAGGGTTCATGTCCTTCAGA-3′.
Assays of neurite formation and viability assays
Neurite extension of neuroblastoma cells BE(2)-C was determined after 7 days of continuous exposure to 10 μ M aRA and 30 or 100 μ M copper. Cells were scored as positive for neurite outgrowth if the neuritic process was longer than the cell body. The percentage of positive cells was counted within each high-powered field, with a total of 300 cells counted in an average of five fields. Each experiment was performed in duplicate on three separate occasions. Cell viability was assessed using Alamar Blue reagent as described earlier (Haber et al, 1999).
siRNA-transient transfection
SH-SY5Y and BE(2)-C cells at approximately 50% confluence were transfected with 30 nM ATP7A siRNA (Smart Pool: Dharmacon, Chicago, IL, USA) or 10 nM RARβ2 siRNA and 10 nM siGENOME non-targeting siRNA as control siRNA (Dharmacon) using Lipofectamine 2000 (Invitrogen) reagent according to the manufacturer's protocols. Following transfection, cells were incubated overnight in normal media, and then treated with 1 μ M aRA or solvent control. Cells were harvested and RNA was extracted 48 or 72 h later. Transfection efficiency and its effect on target gene expression, was assessed by competitive RT–PCR.
Western blot
Cells were harvested in the presence of protease inhibitors by direct lyses in the culture vessel with lyses buffer (RIPA: 100 mM NaCl, 0.5% Sodium deoxycholate, 50 mM Tris-Cl, 0.1% SDS, 1% NP-40) and then transferred to eppendorf tubes. Soluble proteins were analysed by SDS–PAGE as published earlier (Cheung et al, 2003). Membranes were probed with chicken IgY anti-ATP7A which is specific for full-length ATP7A (no. ab13995: Abcam, Cambridge, UK), at a dilution of 1/1000 overnight at 4°C. Membranes were then incubated for 1 h at room temperature with a secondary antibody, HRP conjugated rabbit anti-chicken (no. ab6753: Abcam) at a dilution of 1/2000. Membranes were re-probed with an anti-β-Actin antibody (Pierce, IL, USA) as a loading control, according to methods described earlier (Cheung et al, 2003). Relative protein expression was determined by standardising target protein bands against β-Actin using ImageQuant software (Amersham, NSW, Australia).
Mouse monoclonal antibody anti-Myc-Tag (9B11) was purchased from Cell Signalling Technology Inc. (MA, USA) for detecting RARβ2 full-length and its mutant's expression in BE(2)-C cells. Anti-Myc-Tag antibody for RARβ2 deletion mutants immunoblot was used at a 1 : 1000 dilution in 0.5% defatted milk in a TBST buffer. Rabbit polyclonal Actin antibody (Sigma, St Louis, MO, USA) was used at a 1 : 2000 dilution to normalise for differences in loading. Subsequently, membranes were incubated with horseradish peroxidise, anti-mouse or anti-rabbit secondary antibody (Pierce Biotechnology Inc., IL, USA), which was used at a 1 : 5000 dilution. Chemiluminescent detection was performed using the SuperSignal western blotting kit (Pierce Biotechnology, Rockford, IL, USA), according to the manufacturer's instructions.
Accumulation of copper 64
The accumulation of radioactive copper was analysed using standard techniques (Richardson and Baker, 1990; Arredondo et al, 2006). Briefly, BE(2)-C and SH-SY5Y cells in 35 mm wells were incubated for 2 h at 37°C with 10 μCi ml−1 64Cu, in the form of CuCl2 (ARI, Lucas Heights, NSW, Australia) in DMEM supplemented with 10% fetal calf serum. The cells were then placed on a tray of ice and washed four times with ice-cold PBS to remove any non-specifically bound 64Cu. The internalised and membrane-bound 64Cu were incubated with 1 ml trypsin. After this incubation, the cells were then removed from the wells and transferred to γ-counting tubes. Radioactivity was measured using a γ-counter (Wallac Wizard 3; Turku, Finland). Control experiments in previous investigations have found that this technique is valid for measuring membrane-bound and internalised radioactivity (Karin and Mintz, 1981).
Efflux of copper 64
The release of 64Cu by pre-labelled cells was examined using standard procedures (Richardson and Baker, 1991; Food et al, 2002). Cells in 35 mm wells were labelled with 10 μCi ml−1 64Cu in DMEM containing 10% fetal calf serum for 2 h at 37°C. The 35 mm plates were subsequently placed on a tray of ice and washed four times with ice-cold PBS. The cells were then re-incubated with warm DMEM for incubation periods from 1, 2.5 or 4 h to allow cells to efflux copper into the medium. The overlying medium was then removed and placed into γ-counting tubes. The cells were removed from the 35 mm well plates, either by trypsinisation or direct lyses, and transferred to a separate set of γ-counting tubes. Total 64Cu accumulated by cells prior to efflux was determined for each well by adding CPM for 64Cu effluxed and 64Cu retained in the cells. 64Cu effluxed was expressed as a percentage of total 64Cu accumulated. Estimates of 64Cu accumulated per cell was obtained by standardising total 64Cu accumulated against estimated cell viability (OD).
Results
Growth and tumour-suppressive activity of RARβ2 in neuroblastoma cells
We first determined the effect of RARβ2 expression on the proliferative capacity of neuroblastoma cells in the presence and absence of 10 μ M all-trans-retinoic acid (aRA). We performed a BrdU incorporation assay in two human neuroblastoma cell lines (BE(2)-C and SH-SY5Y) transiently transfected with a siRNA specific for RARβ2, followed by 48 h of aRA treatment. RARβ2 expression was reduced by 60% when compared to control siRNA as measured by gel densitometry in both cell lines (Figure 1A). Cell proliferation was significantly increased, with or without aRA, in both neuroblastoma cell lines following RARβ2 knockdown, compared with cells transfected with a non-specific siRNA control (Figure 1A).
To understand the relationship between RARβ2 protein structure and its effects on cell proliferation in the absence of additional retinoid, we made a series of RARβ2 deletion mutants in a tetracycline-inducible expression (Tet-off) vector with a Myc oligopeptide tag (Figure 1B). The RARβ2 deletion mutant proteins were transiently expressed in BE(2)-C cells, and, an anti-Myc epitope antibody identified the expression of all mutant proteins expected. Cell proliferation was measured by BrdU incorporation after cells were grown in culture medium without doxycycline for 72 h. Overexpression of the full length RARβ2 resulted in significantly decreased cell proliferation compared with empty vector. Most importantly, overexpression of the ABC domain (ΔDEF) of the RARβ2 protein alone was sufficient for the growth inhibitory effects of RARβ2 in BE(2)-C cells. In contrast, overexpression of CDEF (ΔAB) and DEF (ΔABC) domains of RARβ2 did not decrease cell proliferation (Figure 1C).
RARβ regulates the expression of ATP7A in neuroblastoma cells
We have previously identified the copper efflux pump protein, ATP7A, as a retinoid-regulated gene using a cDNA microarray analysis of retinoid-treated neuroblastoma cells (Liu et al, 2005). Here, we examined the hypotheses that RARβ2 may regulate ATP7A, and, that ATP7A may be responsible for the growth suppressive effects of RARβ2 in neuroblastoma cells. We found that transcription of both the full-length ATP7A, and the ATP7A splice variant, NML45, were markedly induced by aRA in BE(2)-C and SH-SY5Y cells after treatment with 10 μ M aRA for 7 days (Figure 2A). Consistent with these findings, ATP7A protein expression was also induced by aRA after 3 days of treatment (Figure 2B). Retinoid-induced upregulation of ATP7A appeared to be specific for neuroblastoma cells, as we could not demonstrate an increase in ATP7A mRNA expression in skin, lung or breast cancer cells following retinoid treatment (Figure 2C). We have also observed that the aRA decreases ATP7A mRNA expression in oestrogen-receptor positive MCF7 cells, but not in MDA-MB-234 and MDA-MB-468 breast cancer cells.
ATP7A is induced by retinoid in neuroblastoma tumour cells. (A) Competitive RT–PCR analysis of cDNA samples from cultured neuroblastoma cells treated with 10 μ M aRA, or a solvent control, for up to 7 days. Abundance of ATP7A transcripts (full length and NML45) present in SH-SY5Y and BE(2)-C cDNA samples relative to β2M neuroblastoma cell lines. (B) Western blot analysis of ATP7A protein expression from cultured neuroblastoma cells treated with 10 μ M aRA, or a solvent control, for up to 7 days. Actin gene was used as a loading control. (C) Competitive RT–PCR analysis of ATP7A mRNA expression in cDNA samples from cultured cancer cells treated for 7 days with 10 μ M aRA, or a solvent control.
We have previously characterised two clonally-derived BE(2)-C cell lines stably overexpressing RARβ2, which exhibited growth suppression in the absence of added retinoid (Cheung et al, 1998). Both RARβ2 clones demonstrated increased ATP7A and NML45 expression, when compared with empty vector transfectants. Interestingly, the levels of the splice variant NML45 does not correlate with the levels of RARβ2 in two different RARβ2 clones (Figure 3A). We next transiently transfected BE(2)-C cells with RARβ2-specific siRNA, treated the cells with 10 μ M aRA for 48 h, and examined RARβ2 and ATP7A expression. Consistent with our previous findings, expression of retinoid-induced RARβ2 and ATP7A was coordinately decreased, compared with retinoid-untreated samples (Figure 3B). Additionally, transiently expressing the RARβ2 full-length and the ΔDEF deletion mutant, significantly increased ATP7A expression (Figure 3B). Thus, we found a close relationship between RARβ2 and ATP7A expression levels, and growth inhibition in several different model systems, supporting the notion that ATP7A may mediate the retinoid ligand-dependent, and -independent, actions of RARβ2.
ATP7A transcription is modulated by RARβ2 in neuroblastoma cells. (A) RT–PCR analysis of RARβ2, full length ATP7A and the NML45 ATP7A mRNA expression in RARβ2 stably transfected BE(2)-C cells, compared to empty vector transfected control cells, (B) In the left panel, Real-time PCR analysis of RARβ2 and ATP7A mRNA expression were measured, after 72 h siRNA transfection in BE(2)-C cells with or without 10 μ M aRA treatment for 48 h. Target gene expression was normalised to the expression of the housekeeping gene β2M. In the right panel, RT–PCR analysis of ATP7A mRNA expression in the full length RARβ2 and the RARβ2 ΔDEF deletion mutant, compared with empty vector transfected BE(2)-C cells. *P<0.05 and **P<0.005 indicate a statistically significant difference compared with control. Error bars indicate s.e.m.
The retinoid anti-cancer effect is mediated by effects on ATP7A and intracellular copper
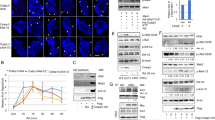
We next examined the hypothesis that ATP7A mediates the effects of RARβ2 on neuroblastoma cells by its effects on intracellular copper metabolism. We measured copper efflux in neuroblastoma cells (BE(2)-C and SH-SY5Y) which were pre-treated with 10 μ M aRA over a time course of 8 days, then loaded with radioactive 64Cu. After 64Cu loading, cells were washed, then incubated in normal growth media to allow copper efflux over a short 4 h time course. In both cell lines there was a higher copper efflux in cells pre-treated with aRA at 4 h (Figure 4A). As the retinoid treatment affects the cell number at the end of the growth period, 64Cu uptake was standardised against cell viability, as assessed by the Alamar Blue assay (Figure 4B). Retinoid treatment resulted in a marked decrease in 64Cu accumulation in both SH-SY5Y and BE(2)-C cells.
Modulation of cellular copper efflux and accumulation by retinoid treatment in neuroblastoma cells. (A) Copper efflux was monitored over 4 h, and is expressed as a percentage of copper uptake in both SH-SY5Y and BE(2)-C cells after 10 μ M aRA treatment for 8 days. (B) SH-SY5Y and BE(2)-C cells were treated for 2, 4 and 8 days with 10 μ M aRA, or with a control treatment, and viability measured using Alamar Blue assay. Cells were then incubated in normal media with 10 μC radioactive 64Cu for 2 h to allow uptake. 64Cu accumulation is expressed as counts per minute, standardised against cell viability. (C) SH-SY5Y cells were transient transfected with an ATP7A siRNA without aRA treatment. ATP7A mRNA expression were measured by RT–PCR. The rate of copper efflux in SH-SY5Y was measured as described in Materials and methods section. (D) BE(2)-C and SH-SY5Y cells were treated with 1 μ M aRA treatment for 24 h, knock-down of ATP7A expression by ATP7A siRNA (left panel) resulted in an increase in cell viability as measured by the Alamar Blue assay (right panel).
To directly examine the role of ATP7A in modulating copper efflux in neuroblastoma cells, we used siRNA specific for ATP7A. Transient transfection of SH-SY5Y cells with an ATP7A siRNA lead to a 36% +/− 0.018 s.e.m. reduction in ATP7A expression and a 27% +/− 0.015 s.e.m. reduction in the rate of copper efflux in retinoid-untreated cells, suggesting that the rate of copper efflux in this cell line was dependent on ATP7A, and, was proportional to the level of ATP7A expression (Figure 4C). There was no effect on BE(2)-C cells. The ATP7A knock-down did not alter the copper accumulation in untreated SH-SY5Y or BE(2)-C cells. We next examined the effect of inhibiting ATP7A expression on the viability of retinoid-treated neuroblastoma cells. Owing to the combined toxicity of siRNA and retinoid treatment, the experiment was limited to 24 h retinoid treatment, and a lower retinoid concentration of 1 μ M aRA. In both SH-SY5Y and BE(2)-C cells, a reduction in ATP7A expression of approximately 30% correlated with an increase in cell viability in the Alamar Blue assay, compared with a non-specific siRNA control (Figure 4D).
We next examined the effects of copper supplementation on the retinoid responses of neuroblastoma cells. BE(2)-C and SH-SY5Y cells were grown according to standard techniques, in the absence of added retinoid and supplemented with 0, 30, 100 and 300 μ M copper treatment for 48 h. Cell proliferation was assessed using the BrdU incorporation assay. Supplementing BE(2)-C with 30, 100 and 300 μ M CuCl2 significantly increased cell proliferation (Figure 5A). None of the concentrations tested increased the proliferation of SH-SY5Y cells (data not shown). BE(2)-C and SH-SY5Y cells were then grown for 7 days with 10 μ M aRA, in the presence or absence of 30 and 100 μ M CuCl2. After 7 days, differentiation was assessed by neurite count. Added CuCl2 significantly reduced neurite formation in BE(2)-C cells over the 7 day retinoid treatment (Figure 5A). Moreover, the copper chelator, Triethylenetetramine (TETA), reduced the viability and proliferative capacity of BE(2)-C cells compared to cells grown without TETA. The effect was completely abrogated when TETA was added with equimolar CuCL2 (Figure 5B). Copper supplementation had no observable effect on retinoid-induced differentiation of SH-SY5Y cells. In both cell lines copper supplementation did not affect the neurite count of cells grown in the absence of the added retinoid (data not shown).
Effect of copper supplementation and depletion in neuroblastoma cells. (A) In the left panel, supplementing with up to 300 μ M CuCl2, over 48 h growth, caused an increase in proliferation of BE(2)-C cells measured by Brdu incorporation. In the right panel, BE(2)-C cells were grown for 7 days with 10 μ M aRA, in the presence or absence of CuCl2. At the end of 7 days, differentiation was assessed by neurite count. (B) Depletion of available copper from the media by addition of the copper chelator Triethylenetetramine (TETA) at 30 or 60 μ M (final concentration) caused a decrease in viability of BE(2)-C cells as measured by Alamar Blue assay. The 60 μ M TETA also resulted in decreased proliferation of BE(2)-C cells as measured by the BrdU assay. Neutralisation of TETA by addition of equimolar CuCl2 abrogated its effects. *P<0.05 indicate a statistically significant difference compared with untreated.
Lastly, we investigated whether a combination of retinoid and copper chelator could increase their anti-cancer activity. Both BE(2)-C and SH-SY5Y neuroblastoma cells were treated with a single agent or a combination of aRA (1 μ M) and TETA (60 μ M) for 72 h. The effect of the copper chelator by itself had only a minor growth inhibitory effect on SH-SY5Y and BE(2)-C cell lines, whereas the combination treatment of retinoid and copper chelator had a marked effect on cell viability and proliferation (Figure 6).
Growth inhibitory effects of combined copper chelator and retinoid treatment. BE(2)-C and SH-SY5Y cells were treated by 1 μ M aRA, 60 μ M TETA or combination of 1 μ M aRA and 60 μ M TETA for 72 h, cell growth was measured by BrdU incorporation and Alamar Blue assay. *P<0.05 and **P<0.005 indicate a statistically significant difference compared with control.
Discussion
A therapeutic role for retinoids in several human cancer types, including neuroblastoma, is well established. However, retinoids are not completely effective anti-cancer agents when used alone; thus, a better understanding of their mechanism of action will lead to more evidence-based retinoid combination therapies. In this study we have demonstrated that RARβ2 has a strong, retinoid-dependent and -independent, anti-proliferative effects on neuroblastoma cells. Furthermore, we have, for the first time, identified a link between the retinoid anti-cancer signal and intracellular copper levels, which has important implications for the understanding of the tumorigenic process and therapy of neuroblastoma.
In human neuroblastoma cells, knocking down RARβ2 expression resulted in a clear increase in cell proliferation, regardless of whether or not exogenous retinoid was added. Conversely, overexpression of RARβ resulted in a strong decrease in cell proliferation. The RARβ2 anti-proliferative effect required only the ABC domain (AF1 and DNA-binding domains), and not the retinoid-binding domain or receptor dimerisation interface. This result strongly suggests that RARβ2 has both retinoid-dependent and -independent anti-cancer functions.
In a previous study using the cDNA microarray, we found that the copper transporter ATP7A was strongly upregulated in neuroblastoma cells in response to retinoid (Liu et al, 2005). In this study, we found that ATP7A protein expression is upregulated in neuroblastoma cells after retinoid treatment. As retinoid treatment did not result in similar upregulation of ATP7A expression in the non-neuronal cell lines, which tested, we suggest that retinoid regulation of ATP7A is tissue type-dependent and may be specific to neuronal cells. We have also shown that ATP7A upregulation can be achieved by ectopic expression of full-length RARβ2 or the RARβ2 ABC domain in the absence of added retinoid. Our data supports the notion that RARβ2, directly or indirectly regulates ATP7A expression in a retinoid-dependent and -independent manner.
During neuronal development, ATP7A expression over time is precisely regulated, and varies according to cell type (Linz and Lutsenko, 2007). Studies of the mouse brain show that ATP7A expression is most abundant in the early postnatal period and decreases from birth to adulthood (Niciu et al, 2006). The mechanism by which RARβ regulates ATP7A expression is unknown. As there are no known RARE in the ATP7A promoter region, it is likely that RARβ regulates ATP7A expression level indirectly. Previous studies focused on the role of ATP7A in Menke's disease, have shown that ATP7A expression and copper plays an important role in neural differentiation and neuritogenesis (El Meskini et al, 2005, 2007; Birkaya and Aletta, 2005). We found an increase in cell viability when ATP7A expression was knocked down in neuroblastoma cells (Liu et al, 2005). The significant correlation between ATP7A and RARβ expression suggests that ATP7A may contribute to the RARβ anti-cancer therapeutic effect.
The role of copper in biological phenomena that involve signal transduction is poorly understood. A cellular model of neuronal differentiation has been utilised to examine the requirement for copper during nerve growth factor (NGF) signal transduction that leads to neurite outgrowth (Birkaya and Aletta, 2005). NGF increases cellular copper content within 3 days, whereas copper chelators reduce the effects of NGF on neurite outgrowth and copper accumulation (Birkaya and Aletta, 2005). Our data supports a model of malignant neural cells requiring higher intracellular copper levels for viability, and that retinoid-induced neuritic differentiation of neuroblastoma cells causes intracellular copper depletion. In non-neuronal cells ATP7A regulates copper homeostasis by translocating copper from the trans-Golgi network to the plasma membrane, in response to an increased copper load. In Menkes disease a defect in the ATP7A protein leads to a reduced transport of copper from the intestine into the circulation and central nervous system, as well as reduced transport of copper into the Golgi apparatus. In our studies, a significant increase in copper efflux, and decrease in copper uptake occurred over 8 days of retinoid treatment. Thus, reduction in cellular copper levels directly contributes to the retinoid therapeutic effect.
In summary, our results indicate that ATP7A is an important component of the RARβ anticancer effect. Most importantly, copper chelators reduce viability and proliferation of neuroblastoma cells. ATP7A and copper are novel potential drug targets that may be exploited in the treatment of neuroblastoma.
References
Alvarez S, Germain P, Alvarez R, Rodriguez-Barrios F, Gronemeyer H, de Lera AR (2007) Structure, function and modulation of retinoic acid receptor beta, a tumor suppressor. Int J Biochem Cell Biol 39: 1406–1415
Arredondo M, Martinez R, Nunez MT, Ruz M, Olivares M (2006) Inhibition of iron and copper uptake by iron, copper and zinc. Biol Res 39: 95–102
Berg WJ, Nanus DM, Leung A, Brown KT, Hutchinson B, Mazumdar M, Xu XC, Lotan R, Reuter VE, Motzer RJ (1999) Up-regulation of retinoic acid receptor beta expression in renal cancers in vivo correlates with response to 13-cis-retinoic acid and interferon-alpha-2a. Clin Cancer Res 5: 1671–1675
Birkaya B, Aletta JM (2005) NGF promotes copper accumulation required for optimum neurite outgrowth and protein methylation. J Neurobiol 63: 49–61
Castillo L, Milano G, Santini J, Demard F, Pierrefite V (1997) Analysis of retinoic acid receptor beta expression in normal and malignant laryngeal mucosa by a sensitive and routine applicable reverse transcription-polymerase chain reaction enzyme-linked immunosorbent assay method. Clin Cancer Res 3: 2137–2142
Cheung B, Hocker JE, Smith SA, Norris MD, Haber M, Marshall GM (1998) Favorable prognostic significance of high-level retinoic acid receptor beta expression in neuroblastoma mediated by effects on cell cycle regulation. Oncogene 17: 751–759
Cheung B, Hocker JE, Smith SA, Reichert U, Norris MD, Haber M, Stewart BW, Marshall GM (1996) Retinoic acid receptors beta and gamma distinguish retinoid signals for growth inhibition and neuritogenesis in human neuroblastoma cells. Biochem Biophys Res Commun 229: 349–354
Cheung B, Yan J, Smith SA, Nguyen T, Lee M, Kavallaris M, Norris MD, Haber M, Marshall GM (2003) Growth inhibitory retinoid effects after recruitment of retinoid X receptor beta to the retinoic acid receptor beta promoter. Int J Cancer 105: 856–867
El Meskini R, Cline LB, Eipper BA, Ronnett GV (2005) The developmentally regulated expression of Menkes protein ATP7A suggests a role in axon extension and synaptogenesis. Dev Neurosci 27: 333–348
El Meskini R, Crabtree KL, Cline LB, Mains RE, Eipper BA, Ronnett GV (2007) ATP7A (Menkes protein) functions in axonal targeting and synaptogenesis. Mol Cell Neurosci 34: 409–421
Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240: 889–895
Food MR, Sekyere EO, Richardson DR (2002) The soluble form of the membrane-bound transferrin homologue, melanotransferrin, inefficiently donates iron to cells via nonspecific internalization and degradation of the protein. Eur J Biochem 269: 4435–4445
Gronemeyer H, Gustafsson JA, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3: 950–964
Haber M, Bordow SB, Gilbert J, Madafiglio J, Kavallaris M, Marshall GM, Mechetner EB, Fruehauf JP, Tee L, Cohn SL, Salwen H, Schmidt ML, Norris MD (1999) Altered expression of the MYCN oncogene modulates MRP gene expression and response to cytotoxic drugs in neuroblastoma cells. Oncogene 18: 2777–2782
Harris ED (2000) Cellular copper transport and metabolism. Annu Rev Nutr 20: 291–310
Karin M, Mintz B (1981) Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem 256: 3245–3252
La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Camakaris J, Mercer JF (1999) Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum Mol Genet 8: 1069–1075
Leid M, Kastner P, Chambon P (1992) Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci 17: 427–433
Linz R, Lutsenko S (2007) Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J Bioenerg Biomembr 39: 403–407
Liu T, Bohlken A, Kuljaca S, Lee M, Nguyen T, Smith S, Cheung B, Norris MD, Haber M, Holloway AJ, Bowtell DD, Marshall GM (2005) The retinoid anticancer signal: mechanisms of target gene regulation. Br J Cancer 93: 310–318
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408
Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK (1995) Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. New Engl JMed 332: 1405–1410
Lutsenko S, Petris MJ (2003) Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol 191: 1–12
Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM (1990) Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 345: 224–229
Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA (2006) Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience 139: 947–964
Norris MD, Bordow SB, Haber PS, Marshall GM, Kavallaris M, Madafiglio J, Cohn SL, Salwen H, Schmidt ML, Hipfner DR, Cole SP, Deeley RG, Haber M (1997) Evidence that the MYCN oncogene regulates MRP gene expression in neuroblastoma. Eur J Cancer 33: 1911–1916
Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud JM (1999) Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst 91: 1059–1066
Reddy MC, Majumdar S, Harris ED (2000) Evidence for a Menkes-like protein with a nuclear targeting sequence. Biochem J 350 (Part 3): 855–863
Richardson DR, Baker E (1990) The uptake of iron and transferrin by the human malignant melanoma cell. Biochim Biophys Acta 1053: 1–12
Richardson DR, Baker E (1991) The release of iron and transferrin from the human melanoma cell. Biochim Biophys Acta 1091: 294–302
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council Australia, the Cancer Council New South Wales and the Cancer Institute New South Wales (GM Marshall, M Norris and M Haber), and by a Cancer Institute New South Wales Early Career Development Fellowship (BB Cheung).
The Children's Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children's Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/.
About this article
Cite this article
Bohlken, A., Cheung, B., Bell, J. et al. ATP7A is a novel target of retinoic acid receptor β2 in neuroblastoma cells. Br J Cancer 100, 96–105 (2009). https://doi.org/10.1038/sj.bjc.6604833
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604833
Keywords
This article is cited by
-
Diagnostic and Dosimetry Features of [64Cu]CuCl2 in High-Grade Paediatric Infiltrative Gliomas
Molecular Imaging and Biology (2023)
-
Cellular and sub-cellular Cu isotope fractionation in the human neuroblastoma SH-SY5Y cell line: proliferating versus neuron-like cells
Analytical and Bioanalytical Chemistry (2019)
-
Synthesis, anti-inflammatory and neuroprotective activity of pyrazole and pyrazolo[3,4-d]pyridazine bearing 3,4,5-trimethoxyphenyl
Medicinal Chemistry Research (2017)
-
Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway
Nature Communications (2016)
-
Retinoic acid signaling and neuronal differentiation
Cellular and Molecular Life Sciences (2015)