Abstract
High-level microsatellite instability (MSI-H) in colorectal cancer accounts for about 12% of colorectal cancers and is typically associated with a dense infiltration with cytotoxic CD8-positive lymphocytes. The role of regulatory T cells that may interfere with the host's antitumoural immune response in MSI-H colorectal cancers has not been analysed yet. Using an antibody directed against the regulatory T-cell marker transcription factor forkhead box P3 (FOXP3), regulatory T cells were examined in 70 colorectal cancers with known MSI status (MSI-H, n=37; microsatellite stable, n=33). In MSI-H colorectal cancers, we found a significantly higher intraepithelial infiltration with FOXP3-positive cells (median: 8.5 cells per 0.25 mm2 vs 3.1 cells per 0.25 mm2 in microsatellite stable, P<0.001), and a significantly elevated ratio of intraepithelial to stromal infiltration (0.05 vs 0.01 in microsatellite stable, P<0.001). CD8-positive cell counts were related positively to the number of FOXP3-positive cells (Spearman's ρ=0.56 and 0.55, respectively). Our results show that the elevated number of CD8-positive lymphocytes found in MSI-H colorectal cancers is paralleled by an enhanced infiltration with CD8-negative FOXP3-positive cells. These data suggest that FOXP3-positive cells may play a role in the regulation of the immune response directed against MSI-H colorectal cancers at the primary tumour site.
Similar content being viewed by others
Main
Colorectal cancers (CRCs) arise through two major molecular pathways. Although the majority of CRCs is hallmarked by large chromosomal alterations (Lengauer et al, 1997), about 10–15% of CRCs are characterised by the high-level microsatellite instability (MSI-H) phenotype that results from defects in the DNA mismatch repair system. The anti-tumoural immune response against CRCs is apparently closely linked to the molecular pathogenesis of these tumours, and MSI-H CRCs typically present with features of a pronounced local immune response, for example a high density of tumour-infiltrating lymphocytes with a high proportion of activated and cytotoxic CD8-positive lymphocytes (Dolcetti et al, 1999; Smyrk et al, 2001; Phillips et al, 2004; Jenkins et al, 2007). In line with the pronounced immunogenicity of MSI-H CRCs, these cancers rarely develop metastases in distant organs and have a comparably good prognosis (Buckowitz et al, 2005; Popat et al, 2005) in spite of a large tumour mass at the primary localisation (Wright et al, 2000).
The immunogenicity of MSI-H CRCs is ascribed to the generation of multiple immunogenic frameshift-derived antigens as a consequence of coding microsatellite mutations that result from mismatch repair deficiency (Linnebacher et al, 2001; Saeterdal et al, 2001; Schwitalle et al, 2008). The presence of these well-defined tumour-specific antigens renders MSI-H tumours as a unique model entity for immunological studies.
The outgrowth of MSI-H CRCs despite the presence of a dense lymphocytic infiltration suggests that several mechanisms interfere with the efficiency of the host's immune response in vivo. For example, immune evasion mechanisms such as impairment or loss of the human leukocyte antigen class I-mediated antigen presentation (Bicknell et al, 1996; Cabrera et al, 2003; Kloor et al, 2005) or the expression of Fas ligand (Okada et al, 2000; Michael-Robinson et al, 2003) are frequent in MSI-H CRCs.
In the recent past, regulatory T (Treg) cells and their role in the suppression of antitumoural immune responses have gained increasing attention. Treg cells represent a heterogeneous group of T cells that are defined on the basis of their ability to control the activation and function of antigen-reactive T cells in the periphery, thereby preventing self-reactivity (reviewed in Knutson et al, 2007). Treg cells can suppress reactivity of tumour antigen-specific T cells in CRC patients (Clarke et al, 2006). The number of Treg cells in the peripheral blood and the tumour itself are increased in patients suffering from gastrointestinal malignancies including CRCs (Ichihara et al, 2003; Sasada et al, 2003; Kono et al, 2006; Loddenkemper et al, 2006; Ling et al, 2007). This suggests that Treg cells may modulate the anti-tumoural immune response in CRC patients.
To date, it is unknown whether the well-established difference between MSI-H and MSS CRCs concerning the infiltration with lymphocytes of the cytotoxic and activated phenotype is paralleled by a difference in Treg cell infiltration. Recently, a novel Treg cell marker, forkhead box P3 (FOXP3), has been described (Fontenot et al, 2003; Hori et al, 2003; Khattri et al, 2003). The expression of FOXP3 in T cells corresponds with immune regulatory function (Hori et al, 2003; Fontenot et al, 2005; Roncador et al, 2005), indicating that the number of FOXP3-positive tumour-infiltrating lymphocytes is representative of the number of potential immune suppressor T cells in the tumour microenvironment (reviewed in Banham et al, 2006). To determine potential differences between Treg cell infiltration between MSI-H and MSS CRCs, we analysed infiltration with FOXP3-positive cells in 70 primary CRC lesions tested for their MSI status.
Materials and methods
Tumour samples and MSI analysis
Colorectal cancer samples were collected in the Heidelberg Centre for Familial Colorectal Cancer as part of a prospective study funded by the German Cancer Aid (Deutsche Krebshilfe) that has been approved by the Institutional Ethics Committee of the University of Heidelberg. Tumours were typed for MSI using the standard NCI/ICG-HNPCC marker panel (Boland et al, 1998) and CAT25 as described earlier (Findeisen et al, 2005). For tumour staging, the UICC/AJCC TNM system was applied (American Joint Committee on Cancer, 1997). Tumours of the patients who had received neo-adjuvant chemotherapy and tumours with a mucinous histology or extensive necrotic areas were excluded from the study. A total number of 37 MSI-H CRCs and 33 MSS CRCs were analysed.
Immunohistochemistry
Tissue sections (2 μm) were prepared from formalin-fixed, paraffin-embedded material and mounted on aminopropylsilane-coated slides (SuperFrost, Menzel, Braunschweig, Germany). After deparaffinisation and rehydration, the slides were boiled in 10 mM citrate buffer (pH 6) for 15 min to retrieve the antigens. Subsequently, the slides were allowed to cool for 30 min.
For the detection of CD3, CD8 and FOXP3 antigens, immunohistochemistry using ABC method was applied. The endogenous peroxidase activity was blocked by incubation with 0.6% H2O2 in methanol for 20 min. The sections were blocked with 10% normal horse serum (Vectastain Elite ABC kit, Vector, Burlingame, USA). For immunostaining, mouse monoclonal antibodies specifically recognising CD3 (1 : 50 dilution, clone PS1, Acris, Heford, Germany), CD8 (1 : 40 dilution, clone 4B11, Novocastra, Newcastle, UK) and FOXP3 (1 : 50 dilution, clone 236A/E7, eBioscience, San Diego, USA) were applied as primary antibodies at 4°C overnight. The slides were incubated with a biotinylated secondary antibody (1 : 50 dilution, horse anti-mouse IgG, Vectastain Elite ABC kit, Vector) for 30 min at room temperature and AB reagent was applied according to the manufacturer's instructions (Vectastain Elite ABC kit). The antigen detection was performed by a colour reaction with 3,3-diaminobenzidine (DAB+ chromogen, DakoCytomation, Glostrup, Denmark). The sections were counterstained with haematoxylin (AppliChem, Germany) and mounted with Aquatex (Merck).
For immunofluorescence staining, slides were co-incubated for 2 h at room temperature with the following monoclonal antibodies: mouse anti-CD3 (1 : 50 dilution, clone PS1, Acris) and rat anti-FOXP3 (1 : 100 dilution, clone PCH101, eBioscience) for CD3/FOXP3 double staining, mouse anti-CD8 (1 : 40 dilution, clone 4B11, Novocastra) and rat anti-FOXP3 (1 : 100 dilution, clone PCH101, eBioscience) for CD8/FOXP3 double staining. Secondary antibodies (goat anti-mouse IgG labelled with Alexa Fluor 488 and donkey anti-rat IgG labelled with Alexa Fluor 594; both from Molecular Probes, Eugene, OR, USA) were applied in a 1 : 50 dilution for 1 h at room temperature. Slides were counterstained with 4′,6-diamidino-2-phenylindol (2 μg ml, Roche, Germany) and mounted with 9.6% Mowiol 4-88 (Roth, Karlsruhe, Germany) in Tris glycerol containing 0.1% DABCO (Roth). Negative controls were prepared by omitting the primary antibodies. In addition, incubation of rat primary antibodies with anti-mouse secondary antibody and vice versa was performed to exclude cross-reactivity of the secondary antibodies.
Microscopic evaluation
Evaluation of the immunohistological stains was performed without knowledge of the MSI status of the tumour or clinical data. For counting and documentation of tumour-infiltrating lymphocytes, five representative fields (i=1 to i=5) of the tumour were chosen from each slide, and the stained cells were counted by means of a 10 × 10 ocular grid at × 200 magnification (observed area 0.25 mm2) using a Leica DMRBE microscope (Leica, Solms, Germany). Each field was subdivided into an epithelial and a stromal compartment, and for both compartments, cellular infiltration (ni) and the compartment's area (number of grid elements, ai) were recorded separately. The relative numbers of stained cells per 0.25 mm2 (n) were calculated for tumour epithelium and stroma using the following formula:

Pictures were taken using a Leica DMRBE microscope (Leica) and a digital camera DFC480 (Leica), or scanned using a Hamamatsu NanoZoomer Digital Pathology system (Hamamatsu, Hamamatsu City, Japan). Immunofluorescence images were calculated presenting each fluorochrome signal with an artificial color: Alexa Fluor 488, green (CD3 and CD8, respectively), Alexa Fluor 594, red (FOXP3), and DAPI, blue (cell nuclei).
Statistical analysis
The correlation between the numbers of FOXP3-positive, CD3-positive and CD8-positive cells infiltrating the tumour compartments was estimated using Spearman's rank correlation coefficient. The exact Mann–Whitney test was used for pairwise comparisons of the location of distributions of quantitative variables. Fisher's exact test was applied for pairwise comparisons of categorical variables. Two-sided tests were used for the pairwise comparisons. A univariate linear regression analysis was used to relate the numbers of FOXP3-positive cells to clinical variables. A result with a P-value lesser than 0.05 was always considered statistically significant. All statistical analyses were performed using R (R Development Core Team, 2007), version 2.6.1 together with the R package exactRankTests, version 0.8-16.
Results
To compare the infiltration with FOXP3-positive cells in CRCs with different MSI status, we analysed 37 MSI-H CRCs and 33 MSS CRCs. At diagnosis, the age of patients with MSI-H CRCs was similar compared with the age of patients with MSS CRCs (median age 51 vs 49 years, respectively; P=0.92). Localisation in the proximal colon was more frequent in MSI-H CRCs than in MSS CRCs (P<0.001). There were no significant differences between the MSI-H and MSS groups concerning TNM or UICC stage distribution. Patient's characteristics are summarised in Table 1.
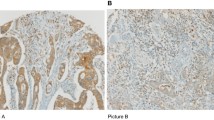
Infiltration of the tumour with FOXP3-positive cells was observed in all CRC specimens. In all tumours, the number of infiltrating FOXP3-positive cells was higher in the tumour stroma than in the epithelium. In addition to the staining for FOXP3, all tumours were stained with antibodies against CD3 and CD8 to assess the overall infiltration with CD3-positive T cells and with CD8-positive T cells. As observed for the FOXP3 stain, all tumours showed infiltration with CD3-positive and CD8-positive lymphocytes, which was higher in the tumour stroma than in the epithelium. The median values for the intraepithelial and stromal infiltration of MSI-H and MSS CRCs with FOXP3-positive, CD3-positive and CD8-positive lymphocytes are summarised in Table 2. The exemplary staining results are displayed in Figure 1.
The comparison of MSI-H and MSS CRCs revealed a higher infiltration of MSI-H CRCs with FOXP3-positive cells. High-level microsatellite instability CRCs showed a trend towards a higher infiltration with FOXP3-positive cells in the tumour stroma (median: 181.5 cells per 0.25 mm2 in MSI-H vs 137.1 cells per 0.25 mm2 in MSS, P=0.06) and a significantly higher intraepithelial infiltration with FOXP3-positive Treg cells (median: 8.5 cells per 0.25 mm2 in MSI-H vs 3.1 cells per 0.25 mm2 in MSS, P<0.001). In addition, the ratio between intraepithelial and stromal FOXP3-positive cells was significantly higher in the MSI-H CRC group (0.05 in MSI-H vs 0.01 in MSS, P<0.001), indicating that in MSI-H CRC, a larger proportion of the FOXP3-positive cells was located in the epithelial compartment of the tumour. A graphical display of FOXP3-positive cell counts is shown in Figure 2. In accordance with previously published studies, we found higher numbers of intraepithelial CD3-positive T cells (median: 60.8 cells per 0.25 mm2 in MSI-H vs 14.1 cells per 0.25 mm2 in MSS, P<0.001) and CD8-positive T cells (median: 32.5 cells per 0.25 mm2 in MSI-H vs 6.3 cells per 0.25 mm2 in MSS, P<0.001) in MSI-H compared with MSS CRCs. In addition, the numbers of CD3-positive T cells (370.8 cells per 0.25 mm2 in MSI-H vs 320.1 cells per 0.25 mm2 in MSS, P=0.06) and CD8-positive cells (107.4 cells per 0.25 mm2 in MSI-H vs 47.2 cells per 0.25 mm2 in MSS P=0.009) infiltrating the tumour stroma were higher in MSI-H CRCs. The comparison of FOXP3-positive with CD8-positive cell counts revealed a positive correlation between the two markers (Spearman's rank correlation coefficient ρ=0.60, 95% confidence interval: 0.43–0.73).
(A) Epithelial counts of FOXP3-positive cells in MSS and MSI-H colorectal cancers. (B) Stromal counts of FOXP3-positive cells in MSS and MSI-H colorectal cancers. (C) The ratio of epithelial-to-stromal FOXP3-positive cell counts in MSS and MSI-H colorectal cancers. Y axis was truncated at 0.4, § represents one extreme value at 0.7. Open circles represent outliers (above 1.5 interquartile ranges), * represent extreme values (above 3 interquartile ranges).
In addition, we evaluated the association of clinicopathological variables with the intratumoural FOXP3-positive cell infiltration. We could not identify a statistically significant influence of age or gender on the overall infiltration of CRC with FOXP3-positive cells, yet a younger age was associated with a lower number of FOXP3-positive cells in the stroma and a higher ratio of epithelial-to-stromal infiltration with FOXP3-positive cells (P=0.07 and P=0.08, respectively). The infiltration with FOXP3-positive cells did not differ significantly between the local tumour stages (T1–T4). In CRC with nodal metastasis (N1/2), the number of stromal FOXP3-positive cells was significantly lower than in CRC without nodal metastasis (N0) (median: 136.9 cells per 0.25 mm2 in N1/2 vs 173.6 cells per 0.25 mm2 in N0, P=0.02), whereas the number of intraepithelial FOXP3-positive cells was not different between both groups (median: 5.4 cells per 0.25 mm2 in N1/2 vs 4.4 cells per 0.25 mm2 in N0, P=0.92). Similarly, tumours that had developed metastases in distant organs (M1) also showed a lower stromal infiltration with FOXP3-positive cells (median: 75.7 cells per 0.25 mm2 in M1 vs 171.6 cells per 0.25 mm2 in M0, P=0.01) compared with tumours without distant metastasis (M0), whereas the intraepithelial infiltration was not different (median: 5.1 cells per 0.25 mm2 in M1 vs 4.5 cells per 0.25 mm2 in M0, P=0.72).
Recent studies indicated that FOXP3 may be expressed transiently in CD8-positive effector T cells upon activation (Shevach, 2006; Baron et al, 2007). To further characterize the phenotype of FOXP3-positive cells detected in CRC lesions, immunofluorescence double staining was performed using antibodies specific for CD8/FOXP3, and CD3/FOXP3 as a control. Five CRC lesions showing high numbers of intraepithelial FOXP3 cells were selected for the analysis. Immunofluorescence stainings showed that all detectable FOXP3-positive cells were negative for CD8. In contrast, nuclear FOXP3 signals were regularly accompanied by membrane-bound CD3 staining. Exemplary images of immunofluorescence analysis are shown in Figure 3.
Discussion
The role of Treg cells as suppressors of the host's antitumoural immune response has gained considerable interest in the recent past. The establishment of FOXP3 as a marker for Treg cells allowed for a phenotypic characterisation of the otherwise functionally defined group of Treg cells. Recent studies indicate that FOXP3 expression is not necessarily linked to a regulatory or suppressor phenotype in T cells, for example transient FOXP3 expression has been reported in activated CD8-positive effector T cells (Shevach, 2006; Baron et al, 2007). Therefore, it may be hypothesised that FOXP3 expression detected in intraepithelial lymphocytes might be ascribed to locally activated CD8+ cytotoxic T cells, particularly in MSI-H CRCs that are characterized by a high density of CD8-positive tumour-infiltrating T cells. Immunofluorescence double stainings for CD8/FOXP3 and CD3/FOXP3 as a control showed that no cells simultaneously expressing CD8 and FOXP3 were detectable in these lesions. Together with reports from the literature that tumour-infiltrating CD4-positive T cells can be detected in MSI-H as well as in MSS CRC stroma and at lower number also in the CRC epithelium (Dolcetti et al, 1999), these data show that the vast majority of FOXP3-positive T cells infiltrating CRC stroma and epithelium represent CD4-positive cells and not activated CD8-positive effector cells.
Initial studies on CRC specimens reported an elevated number of Treg cells in CRC compared with healthy colonic mucosa (Loddenkemper et al, 2006; Ling et al, 2007). However, Treg cell numbers varied between the tumour specimens; and there is little information about the influence of clinicopathological characteristics of the tumour on intratumoural Treg cell density. It is known from the literature that the number of tumour-infiltrating lymphocytes with a cytotoxic potential in CRC depends on the MSI status of the tumour. In analogy, one may hypothesise that MSI status may also have an influence on the density of tumour-infiltrating Treg cells.
Although a recent study has examined FOXP3 transcript levels in a series of CRCs that had been typed for MSI status (Le Gouvello et al, 2008), this study is the first to systematically analyse the infiltration with FOXP3-positive cells in CRC in dependence of MSI status. Although Le Gouvello et al (2008) observed higher expression levels of FOXP3 mRNA in MSS CRCs compared with MSI-H CRCs, this study detected an enhanced infiltration of FOXP3-postive cells in MSI-H CRCs by immunohistochemistry. This discrepancy might be attributed to the different methodology applied in the studies, and might in part reflect the notion that FOXP3 transcript may be present without the presence of FOXP3 protein (Yamamoto et al, 2008). In addition, the analysis of mRNA levels does not allow for the attribution of FOXP3 expression to specific cell types present in the tumour and may be influenced, for example, by tumour cells expressing FOXP3 mRNA (Hinz et al, 2007).
For our analysis, we chose to differentiate between intraepithelial and stromal infiltration, considering an earlier observation that the prognostic significance of lymphocyte infiltration was mainly related to the number of intraepithelial lymphocytes (Naito et al, 1998; Sato et al, 2005). In our collection, we observed a significantly higher number of FOXP3-positive Treg cells in the stroma compared with the epithelial compartment of the tumour, which is in accordance with the results reported by Loddenkemper et al (2006). However, in contrast to the previously reported absence of Treg cells in tumour epithelium (Ling et al, 2007), intraepithelial FOXP3-positive cells, although sparse in some tumours, were detected in all but one of the analysed lesions.
The comparison of MSI-H and MSS CRCs revealed a significantly higher number of intraepithelial FOXP3-positive lymphocytes in MSI-H compared with MSS CRCs and a trend towards a higher infiltration with these cells in the tumour stroma of MSI-H CRCs. Also in MSI-H CRCs, a higher proportion of the tumour-infiltrating FOXP3-positive cells were located in the epithelium, as indicated by a higher ratio of epithelial-to-stromal infiltration in the MSI-H group. This indicates that the previously known difference between MSI-H and MSS CRCs concerning overall lymphocytic infiltration as well as the infiltration with CD3-positive and CD8-positive T cells also extends to a differential infiltration with FOXP3-positive Treg cells. Moreover, a close association of FOXP3-positive cell counts with numbers of CD8-positive cells was shown (Spearman's rank correlation).
In addition, we looked at several other clinicopathological parameters that may be associated with Treg cell counts, although we concede that the number of tumours included in the study limits the power of the statistical analysis. No significant difference in infiltration with FOXP3-positive cells could be detected between tumours of different local tumour stages (T1–T4), potentially reflecting the limited number of tumour samples in each group. The density of FOXP3-positive cells in tumour stroma was significantly higher in unmetastasised CRCs compared with tumours with lymph node or distant metastases. These data are in accordance with the findings of Loddenkemper et al, who reported a significantly higher Treg infiltration in limited than in metastatic CRC that was mainly caused by Treg infiltrating the tumour stroma. In contrast, no difference in intraepithelial infiltration was observed. Two studies (Atreya et al, 2007; Ling et al, 2007) failed to find an association of intratumoural FOXP3-positive Treg cells or FOXP3 mRNA expression with the presence or absence of metastases. At present, the significance of Treg cell infiltration for the progression of CRC remains unclear and warrants further investigation. Similarly, the association of Treg cell counts with overall or disease-free survival was not feasible in our collection of patients. Concerning the mechanism underlying the increased numbers of tumour-infiltrating FOXP3-positive cells observed in CRCs of the MSI-H group, it is interesting to note that dendritic cells can expand Treg populations (Nagorsen et al, 2007), thus potentially explaining an increased frequency of FOXP3-positive cells in MSI-H CRCs as a consequence of a pronounced immune response observed in these lesions. The mechanisms leading to the observation of enhanced FOXP3-positive cell counts in MSI-H CRCs and its clinical significance need to be addressed in future studies.
In summary, this is the first study systematically examining the association of mismatch repair deficiency and infiltration with FOXP3-positive cells. Our results suggest that the density of Treg cells infiltrating CRCs is significantly higher in MSI-H compared with MSS CRCs, thus paralleling the enhanced number of CD8-positive cells in these tumours. A dense infiltration of MSI-H CRCs with FOXP3-positive cells may play a role in local MSI-H tumour growth in the presence of potentially cytotoxic T cells in the local tumour environment.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
American Joint Committee on Cancer (1997) AJCC Cancer Staging Manual, 5th edn. Lippincott-Raven: Philadelphia
Atreya I, Schimanski CC, Becker C, Wirtz S, Dornhoff H, Schnuerer E, Berger MR, Herr W, Galle PR, Neurath MF (2007) The T-box transcription factor eomesodermin controls CD8+ T cell activity and lymph node metastasis in human colorectal cancer. Gut 56: 1572–1578
Banham AH, Powrie FM, Suri-Payer E (2006) FOXP3+ regulatory T cells: current controversies and future perspectives. Eur J Immunol 36: 2832–2836
Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Türbachova I, Hamann A, Olek S, Huehn J (2007) DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 37: 2378–2789
Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P (1996) Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol 6: 1695–1697
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58: 5248–5257
Buckowitz A, Knaebel HP, Benner A, Blaker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M (2005) Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer 92: 1746–1753
Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F (2003) Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens 61: 211–219
Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ (2006) CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE 1: e129
Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154: 1805–1813
Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, Dondog B, Pawlita M, Dippold W, Wagner R, Gebert J, von Knebel Doeberitz M (2005) T25 repeat in the 3′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res 65: 8072–8078
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY (2005) Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22: 329–341
Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C, Ungefroren H, Saeger HD, Klöppel G, Kabelitz D, Kalthoff H (2007) Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 67: 8344–8350
Hori S, Nomura T, Sakaguchi S, Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061
Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H (2003) Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 9: 4404–4408
Jenkins MA, Hayashi S, O’Shea AM, Burgart LJ, Smyrk TC, Shimizu D, Waring PM, Ruszkiewicz AR, Pollett AF, Redston M, Barker MA, Baron JA, Casey GR, Dowty JG, Giles GG, Limburg P, Newcomb P, Young JP, Walsh MD, Thibodeau SN, Lindor NM, Lemarchand L, Gallinger S, Haile RW, Potter JD, Hopper JL, Jass JR, Colon Cancer Family Registry (2007) Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 133: 48–56
Khattri R, Cox T, Yasayko SA, Ramsdell F (2003) An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 4: 337–342
Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M (2005) Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res 65: 6418–6424
Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H (2006) CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 55: 1064–1071
Knutson KL, Disis ML, Salazar LG (2007) CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother 56: 271–285
Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, Sobhani I (2008) High prevalence of Foxp3 and IL-17 in MMR-proficient colorectal carcinomas. Gut 57 (6): 772–779
Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386: 623–627
Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB, Banham AH, Cerundolo V (2007) Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun 7: 7
Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M (2001) Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 93: 6–11
Loddenkemper C, Schernus M, Noutsias M, Stein H, Thiel E, Nagorsen D (2006) In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J Transl Med 4: 52
Michael-Robinson JM, Pandeya N, Cummings MC, Walsh MD, Young JP, Leggett BA, Purdie DM, Jass JR, Radford-Smith GL (2003) Fas ligand and tumour counter-attack in colorectal cancer stratified according to microsatellite instability status. J Pathol 201: 46–54
Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C (2007) Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med 5: 62
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58: 3491–3494
Okada K, Komuta K, Hashimoto S, Matsuzaki S, Kanematsu T, Koji T (2000) Frequency of apoptosis of tumor-infiltrating lymphocytes induced by fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res 6: 3560–3564
Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S (2004) Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg 91: 469–475
Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23: 609–618
R Development Core Team (2007) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria
Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH (2005) Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol 35: 1681–1691
Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A, Gaudernack G (2001) Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA 98: 13255–13260
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98: 1089–1099
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102: 18538–18543
Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M (2008) Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 134: 988–997
Shevach EM (2006) From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25: 195–201
Smyrk TC, Watson P, Kaul K, Lynch HT (2001) Tumour-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91: 2417–2422
Wright CM, Dent OF, Barker M, Newland RC, Chapuis PH, Bokey EL, Young JP, Leggett BA, Jass JR, Macdonald GA (2000) Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 87: 1197–1202
Yamamoto M, Tsuji-Takayama K, Suzuki M, Harashima A, Sugimoto A, Motoda R, Yamasaki F, Nakamura S, Kibata M (2008) Comprehensive analysis of FOXP3 mRNA expression in leukemia and transformed cell lines. Leuk Res 32: 651–658
Acknowledgements
This study was granted by the Deutsche Krebshilfe (German Cancer Aid). The excellent technical assistance of Beate Kuchenbuch is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Michel, S., Benner, A., Tariverdian, M. et al. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 99, 1867–1873 (2008). https://doi.org/10.1038/sj.bjc.6604756
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604756
Keywords
This article is cited by
-
Immunomodulatory effects of iTr35 cell subpopulation and its research progress
Clinical and Experimental Medicine (2024)
-
The M2 macrophages infiltration of sebaceous tumors is linked to the aggressiveness of tumors but not to the mismatch repair pathway
Journal of Cancer Research and Clinical Oncology (2023)
-
Intratumoral regulatory T cells from colon cancer patients comprise several activated effector populations
BMC Immunology (2021)
-
Clinicopathological characterization and prognostic implication of FOXP3 and CK19 expression in papillary thyroid carcinoma and concomitant Hashimoto's thyroiditis
Scientific Reports (2020)
-
ST2 and regulatory T cells in the colorectal adenoma/carcinoma microenvironment: implications for diseases progression and prognosis
Scientific Reports (2020)