Abstract
Carbonic anhydrase 9 (CA9) is a protein to be upregulated under exposure to hypoxic conditions. Hypoxic conditions are known to be associated with resistance to chemotherapy and radiotherapy, and with poor cancer prognosis. We examined CA9 expression in surgical specimens from oesophageal squamous cell carcinoma (ESCC) patients (n=127) using immunohistochemistry and real-time RT–PCR. We also examined CA9 expression and cell proliferation in ESCC cell lines (TE-2, TE-8 and TE-15) and an immortalised human oesophageal cell line (CHEK-1) using real-time RT–PCR, Western blotting, ELISA and MTT assay. Immunohistochemistry, high expression of CA9 was found in 63 of the 127 primary tumour specimens and was correlated with poor outcome (P=0.0003) and more aggressive/less favourable clinicopathological parameters (tumour size (P=0.0235), tumour depth (P<0.0001), regional lymph node metastasis (P=0.0031), distant lymph node metastasis (P=0.0077), stage (P<0.0001) and blood vessel invasion (P=0.006)). In vitro, CA9 expression in cultured cells and culture medium was also induced by hypoxia (P<0.01). CA9 is correlated with poor prognosis and malignant phenotype in patients with ESCC, and was upregulated by hypoxia. It is suggested that control of CA9 expression might improve the effectiveness of chemotherapy and radiotherapy in ESCC.
Similar content being viewed by others
Main
Oesophageal cancer is a common malignant neoplasm throughout the world. Despite recent improvements in surgical techniques, chemotherapy and radiation treatment, the prognosis for patients with advanced disease is still unsatisfactory (Collard et al, 2001; Ando et al, 2003). New approaches to increase the effectiveness of chemotherapy and radiotherapy need to be developed to improve the prognosis of oesophageal cancer.
A hypoxia tumour environment is known to be associated with resistance to chemotherapy and radiotherapy, and with more malignant tumour phenotypes that show increased invasiveness, greater metastatic potential and poor survival (Gray et al, 1953; Brizel et al, 1996; Hockel et al, 1996). Clinical studies have shown a strong correlation between low pretreatment tumour pO2 and poor tumour control and overall survival in patients with head and neck squamous cell carcinoma (Brizel et al, 1999; Nordsmark et al, 2003).
Carbonic anhydrase 9 (CA9), such as hypoxia-inducible factor 1α (HIF-1α), is known to be a marker of hypoxia, but its role in a hypoxic tumour microenvironment is still unclear (Wykoff et al, 2000). The carbonic anhydrase (CA) family of zinc metalloenzymes include at least 14 different isoforms in mammals. Some of these isozymes are cytosolic (CA1, CA2, CA3, CA7, CA13) and others are membrane-bound (CA4, CA9, CA12 and CA14), whereas CA 5 is mitochondrial and CA6 is secreted into saliva and milk. Three cytosolic acatalytic forms are also known (CARP8, CARP10 and CARP11) (Pastorekova et al, 2004). CA is involved in the reversible hydration–dehydration of carbon dioxide, CO2+H2O↔HCO3−+H+. The CA family plays a variety of roles in many physiologic processes, including pH regulation and maintenance of ionic equilibrium (Maren, 1967; Tashian, 1989). Two of the carbonic anhydrases, CA9 and CA12, are transmembrane isozymes that are highly expressed in some tumours, and may be implicated in acidification of the extracellular milieu surrounding cancer cells, thus creating a microenvironment conducive to tumour growth and spread (Pastorek et al, 1994; Ivanov et al, 1998; Türeci et al, 1998; Parkkila et al, 2000).
CA9 expression has been associated with poor prognosis in patients with lung, breast, cervical, brain, and renal cancer (Chia et al, 2001; Giatromanolaki et al, 2001; Loncaster et al, 2001; Bui et al, 2003; Brennan et al, 2006; Haapasalo et al, 2006) and with resistance to chemotherapy in head and neck cancers (Koukourakis et al, 2001). It has been reported that CA9 expression is an independent prognostic marker in oesophageal and gastric adenocarcinomas (Driessen et al, 2006), but the relationship between prognosis and CA9 expression in oesophageal squamous cell carcinoma (ESCC) remains controversial. In vitro studies have indicated that CA9 plays a role in the growth and survival of breast carcinoma cells (Robertson et al, 2004); but its precise function in normal and cancerous tissues remains unclear.
The purpose of this study was to investigate CA9 expression in patients with ESCC, and to confirm changes in the expression level of CA9 in ESCC cell lines and culture medium under hypoxic conditions.
Materials and methods
Clinical samples
This study was performed on 127 patients with ESCC who underwent potentially curative surgery without preoperative therapy at the Department of General Surgical Science, Gunma University Graduate School of Medicine, between 1983 and 2004. Tumour stage was classified according to the sixth edition of the tumour-node-metastasis classification of the International Union against Cancer (UICC) (Sobin and Witteking, 2002). All patients signed informed consent forms in accordance with our institutional guidelines. Information on gender, age, stage of disease, and histopathologic factors was abstracted from medical records.
Before analysis, the resected specimens were fixed with 10% formaldehyde, embedded in paraffin blocks, cut into 4-μm thick sections, and mounted on glass slides. Paraffin-embedded sections were acquired from all 127 patients (112 males and 15 females). The median age of these patients was 62 years (range, 40–79 years), and the median survival time was 30.2 months (range, 1–220 months).
Immunohistochemistry
Immunohistochemical staining of the sections for CA9 expression was performed using the standard streptavidin–biotin peroxidase complex method, as described earlier (Kato et al, 2002; Fukai et al, 2005). Each 4-μm thick section was deparaffinised, rehydrated and incubated with fresh 0.3% H2O2 in methanol for 30 min at room temperature to block endogenous peroxidase activity. After rehydration through a graded series of ethanol concentrations, the sections were autoclaved in 10 mM citrate buffer (pH 6.0) at 120°C for 3 min, and then cooled to 30°C. After rinsing in 0.1 M phosphate-buffered saline (PBS; pH 7.4), non-specific binding sites were blocked by incubation with 10% normal goat serum for 30 min. The sections were then incubated at 4°C overnight with a rabbit polyclonal antibody (Novus Biologicals Inc., Littleton, USA) at a dilution of 1 : 2500 in PBS containing 1% bovine serum albumin. The sections were then washed in PBS, incubated with biotinylated anti-rabbit IgG for 30 min at room temperature, and finally incubated in streptavidin–biotin peroxidase complex solution (Nichirei Co., Tokyo, Japan). The chromogen, 3,3′-diaminobenzidine tetrahydrochloride, was applied as a 0.02% solution containing 0.005% H2O2 in 50 mM ammonium acetate–citrate acid buffer (pH 6.0). Finally, the sections were lightly counterstained with Mayer’s haematoxylin and mounted. Normal gastric mucosa served as a positive control.
Scoring of CA9 protein expression
CA9 expression was evaluated by calculating an immunostaining score, which was the product of the intensity score and the staining rate. Only membranous staining was considered positive. We evaluated tumours at their surface (n=121), centre (n=127) and invasive front (n=125). In accordance with the reported criteria (Chia et al, 2001; Driessen et al, 2006), the intensity score was based on the estimated staining intensity (0, no staining (Figure 1A); 1, weak (Figure 1B); 2, moderate (Figure 1C); 3, strong (Figure 1D)), and the staining rate was defined as the percentage of tumour cells that showed positive staining for CA9 (0–100%). The final immunostaining score (IHC score: 0–300) was calculated as the product of the staining intensity and the staining rate for each CA9-positive tumour. The tumours were considered to have high CA9 expression if the final score exceeded the median score (Driessen et al, 2006).
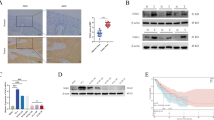
Photomicrographs (magnification= × 200) of oesophageal squamous cell carcinoma and gastro-oesophageal junction immunohistochemically stained for carbonic anhydrase 9 (CA9). (A) The normal gastric mucosa was used as a positive control. The normal oesophageal mucosa was not stained for CA9; (B) weak staining in the tumour; (C) moderate staining in the tumour; (D) strong staining in the tumour.
RNA isolation and cDNA synthesis
Total RNA was extracted from fresh-frozen sections and cell lines using an RNeasy Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Clinical fresh-frozen sections were taken from peripheral tumour areas. The quantity of isolated RNA was measured using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware). Template cDNA was synthesised from 13.5 μg of total RNA using an Omniscript Reverse Transcriptase kit (Qiagen), random primer (hexadeoxyribonucleotide mixture) (TaKaRa, Shiga, Japan) and ribonuclease inhibitor (Porcine liver) (TaKaRa). Total RNA was reverse-transcribed with four units of Omniscript Reverse Transcriptase in a reaction volume of 20 μl (60 min at 37°C, 5 min at 93°C, and finally on ice). The resulting cDNA samples were stored at −30°C until analysis.
Real-time reverse transcriptase–polymerase chain reaction
Real-time reverse transcriptase (RT)–PCR analyses were performed on an ABI Prism 7000 Sequence detection system (Applied Biosystems, Foster City, CA, USA). The standard reaction volume was 20 μl and contained 1 × SYBR Green PCR Master Mix (ABI), 2.0 μl of cDNA template, and both forward and reverse primers at 10 μ M. The initial PCR denaturation step was performed for 5 min at 95°C, followed by 40 cycles of 60 s at 95°C (melting) and 60 s at 60°C (annealing/elongation). All reactions were performed in duplicate. The data were normalised to an internal control gene, β-actin, to control for the amount of RNA in the preparation.
Oligonucleotide primers for CA9 gene amplification by RT–PCR
The oligoribonucleotide primers for CA9 were sense primer 5′-CCTCAAGAACCCCAGAATAATGC-3′; antisense primer 5′-CCTCCATAGCGCCAATGACT-3′, and those for β-actin were: sense primer 5′-CTCCTCCTGAGCGCAAGTACTC-3′; antisense primer 5′-TCCTGCTTGCTGATCCACATC-3′. The amplification was performed for 30 cycles of 30 s at 94°C, 30 s at 62°C (55°C for β-actin) and 30 s at 72°C. Primers for CA9 and β-actin were used with reference to previously published assays (Furuya et al, 2004; Simi et al, 2006).
Cell lines
Three human ESCC cell lines and one immortalised human oesophageal cell line were used: TE-2, TE-8, TE-15 and immortalised human oesophageal cell line (CHEK-1), respectively. TE-2, TE-8 and TE-15 were kindly provided by Dr T Nishihira, Institute of Development, Aging and Cancer, Tohoku University School of Medicine, Sendai, Japan. CHEK-1 cells were kindly provided by Dr H Matsubara. CHEK-1 was established by transfection of human papillomavirus type 16 E6/E7 into primary cultures of human oesophageal keratinocytes (Sashiyama et al, 2002). All cancer cell lines were derived from ESCC with varying degrees of differentiation (Nishihira et al, 1993). TE cell lines and CHEK-1 cells were cultured in RPMI-1640 medium (Sigma Chemical Co., St Louis, MO, USA) supplemented with 10% fetal bovine serum and antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin). All cell lines were cultured to 70–80% confluence.
Parallel incubation was performed on aliquots of cells under normoxic (humidified air with 5% CO2) and hypoxic conditions. Hypoxic conditions were generated in the APM-30D incubator (ASTEC Inc., Fukuoka, Japan) with 1% O2, 5% CO2 and balance N2, after incubation in normoxic conditions for 24 h.
Western blot analysis
Lysates from exponentially growing cell lines were prepared in buffer (20 mM Tris-HCl, pH 7.6, 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 1% aprotinin, 1 mM phenylmethylsulphonyl fluoride and 1 mM sodium vanadate). The protein concentration was determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Protein (40 μg) from each cell line was resuspended in a sodium dodecyl sulphate (SDS) sample buffer (100 mM Tris-HCl, pH 8.8, 0.01% bromophenol blue, 36% glycerol and 4% SDS) containing 1 mM dithiothreitol, boiled for 10 min and applied to a 5–20% gradient Ready-Gel (Bio-Rad, Tokyo, Japan). Proteins were electrotransferred to a Hybond-enhanced chemiluminescence nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). Proteins were immunoblotted using anti-CA9 (Novus Biologicals Inc.) The bands were detected using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). For re-blotting, membranes were stripped according to the manufacturer’s protocol. An anti-β-actin antibody (Sigma Chemical Co.) served as a control. Horizontal scanning densitometry was performed using acquisition into Adobe Photoshop (Apple Inc., Cupertino, CA, USA) and results were analysed by Quantity One (Bio-Rad).
ELISA
The CA9 concentration in the cell culture supernatants was determined by ELISA using a Quantikine® Human Carbonic Anhydrase IX/CA9 Immunoassay Kit (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions. The samples were collected from supernatants of cell lines that had been cultured under normoxic and hypoxic conditions. After centrifugation at 1500 r.p.m., the samples were thawed at 4°C overnight. Then, 50 μl of buffered protein base and 100 μl of sample were added to the wells of a microtitre plate coated with an anti-CA9 mouse monoclonal antibody and incubated for 2 h at room temperature on a horizontal orbital microplate shaker. The plate was washed four times, and 200 μl of conjugated antibody solution was then added. After further incubation, the plate was washed again, 200 μl of substrate solution was added, and a third incubation was carried out for 30 min at room temperature, with protection from light. After the addition of 50 μl of stop solution, colour development was determined immediately at 450 nm using a microtitre plate reader. All samples were assayed three times in a blinded manner, and the mean was used for data analysis.
In vitro proliferation assay
Cell proliferation was measured in ESCC cells using the MTT tetrazolium assay. Briefly, ESCC cells (2.5 × 103 per well) were plated in 96-well plates, 100 μl for each well. After initial cell seeding, the WST-1 assay (Dojin, Tokyo, Japan) was performed, and 10 μl of the cell counting solution was added to each well of the plates, followed by incubation in a humidified 5% CO2 atmosphere at 37°C for 3 h. The formazan was dissolved in 90 μl per well 1 N HCl, and the absorbance of the solution was read at 450 nm using a microtitre plate reader (Becton Dickinson, Franklin Lakes, NJ, USA). All experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using the χ2 test, Fisher’s exact test and Mann–Whitney U-test. Survival curves were calculated by the Kaplan–Meier method and analysis was carried out using the log-rank test. Prognostic factors were examined by univariate and multivariate analyses using a Cox proportional hazards model. Differences at P<0.05 were considered significant. For statistical analyses, we used Stat View Version 5.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Clinical samples
Expression of CA9 protein in ESCC
Staining for CA9 in the normal oesophageal squamous epithelium was negative (Figure 1A), but it was clearly positive in the gastric mucosa used as a positive control (Figure 1A). In cancer cells, CA9 staining was seen mainly in the plasma membrane, and at the border of tumours only the cancerous lesion showed positive staining (Figure 1B–D). In the tumour centre, 76 (59.8%) of the 127 primary tumour specimens showed CA9 positivity. In the tumour surface, 39 (32.2%) of the 121 primary tumour specimens showed CA9 positivity, whereas in the tumour-invasive front, 28 (22.4%) of 125 primary tumour specimens showed CA9 positivity.
Correlations between CA9 expression and clinicopathological findings
For examination of the tumour centre, we divided the tissue sections into a high group and a low group, on the basis of their IHC scores (range, 0–250.2; median, 12.8; mean, 46.7). A median immunostaining score >12.8 was considered to indicate high CA9 expression. Sixty-three of the 127 patients were classified in the high CA9 expression group and the other 64 patients were classified in the low expression group. This classification was in agreement with earlier reports (Chia et al, 2001; Driessen et al, 2006). The correlation between the clinicopathologic characteristics of the ESCC patients and tumour centre CA9 expression is summarised in Table 1. CA9 expression was correlated with tumour size (P=0.0235), tumour depth (P<0.0001), regional lymph node metastasis (P=0.0031), distant lymph node metastasis (P=0.0077), stage (P<0.0001) and blood vessel invasion (P=0.006). However, there was no significant association between CA9 expression and other factors, such as age, gender, histological differentiation, tumour location and lymphatic invasion. We did scoring for all three parts (tumour surface, tumour centre and invasive front) using the method that we described in the paper. There were no associations between the relevant clinicopathological parameters and CA9 staining assessed at the tumour surface or invasive front.
Prognostic significance of CA9 expression
The survival rate of patients with tumours showing low CA9 expression was significantly higher than that of patients with tumours showing high CA9 expression (P=0.0003; Figure 2). The 5-year survival rate for patients with low CA9 expression was significantly higher than that of patients with high CA9 expression (66.5 vs 33.3%). In univariate Cox proportional hazards analysis, primary tumour (T), regional lymph node metastasis (N), distant metastasis (M), TNM stage (S) and CA9 expression were the strongest prognostic factors for cancer-specific survival. However, tumour CA9 expression in ESCC patients was not identified as an independent prognostic factor in multivariate survival analysis using Cox proportional hazards analysis (Table 2).
Association of carbonic anhydrase 9 (CA9) tumour expression with outcome in 127 patients with oesophageal squamous cell carcinoma. Cancer-specific survival rates at 5 years after surgery were 66.5% for the CA9 low expression group and 33.3% for the CA9 high expression group. Kaplan–Meier curve is shown (P=0.0003, log-rank test).
CA9 mRNA expression determined by real-time RT–PCR
To confirm CA9 mRNA expression, we analysed 35 clinical samples, including normal and tumour tissue. All samples quantified values used to calculate CA9/β-actin expression ratios. In this group of paired samples, CA9 mRNA expression levels were significantly higher in tumour tissues (0.022±0.038) (mean±s.d.) than in normal tissues (0.009±0.016). This resulted in a significant difference in the mRNA expression level between tumour and normal tissues (P=0.0019; Figure 3).
Cell lines
Expression of CA9 mRNA cell lines during normoxia and hypoxia
We initially investigated the mechanism of CA9 regulation in ESCC during hypoxia by examining mRNA expression. We cultured the cell lines TE-2, TE-8 and TE-15 for 24 h under hypoxic conditions (1% O2). We then compared CA9 mRNA levels between hypoxic and normoxic conditions using RT–PCR. In all three cell lines, expression of CA9 mRNA was upregulated under hypoxic conditions (Figure 4).
Expression of CA9 in ESCC cell line TE-2 under hypoxic conditions
We compared the temporal expression of CA9 mRNA in the ESCC cell line TE-2 and in the CHEK-1 under hypoxic conditions. In TE-2 cells, the ratio between CA9 mRNA expression during hypoxia relative to normoxia increased markedly with time (Figure 5A). We used Western blotting to examine the expression of CA9 protein in the TE-2 cells. At the protein level, expression of CA9 increased with time under hypoxic but not normoxic conditions (Figure 5B). In CHEK-1 cells, the expression of CA9 protein did not increase under either normoxic or hypoxic conditions (data not shown). This confirmed that CA9 protein was induced by hypoxia. We also measured the expression of CA9 protein in the cell culture medium using ELISA and, interestingly, we found that CA9, which is a membrane protein, was present in the cell culture medium. The level of CA9 in the culture medium under hypoxic conditions was increased significantly when compared with that under normoxic conditions (P<0.01; Figure 5C).
In vitro assay for CA9 induced by hypoxia in an oesophageal squamous cell carcinoma line (TE-2) and in culture medium. (A) Comparison of CA9 mRNA expression in TE-2 cells and in the immortalised human oesophageal cell line (CHEK-1). CA9 expression was measured by real-time RT–PCR; (B) Expression of CA9 protein in TE-2 cells was measured by western blotting. Expression of CA9 protein was higher under hypoxic conditions; (C) Expression of CA9 protein in culture medium was significantly induced under hypoxic conditions (P<0.01). All examinations were done in triplicate. (N, normoxic; H, hypoxic).
Proliferation of ESCC cell lines under hypoxic conditions
We measured cell proliferation in two ESCC cell lines (TE-2 and TE-15), under hypoxic conditions, using the MTT assay. Each sample was measured three times. Both TE-2 and TE-15 cells proliferated significantly more under hypoxic than under normoxic conditions (P<0.01; Figure 6). In addition, the tendency for cell proliferation resembled a change of CA9 expression shown by ELISA.
Discussion
We investigated the expression of CA9 in ESCC at both the protein and mRNA levels, and found that CA9 protein was expressed in tissue samples from 76 of 127 (59.8%) tumours. CA9, similar to HIF-1 (Pantuck et al, 2003), is a member of the hypoxia-induced pathway and is thought to be expressed specifically around the tumour centre, where hypoxic conditions prevail. We therefore performed CA9 scoring for three parts (tumour surface, centre and invasive front), but only the scores from the tumour centre were included in the statistical analyses. Our results reflected those obtained in studies of other solid tumours. Expression of CA9 has been reported in 48% of breast cancers (Chia et al, 2001), 79% of cervical cancers (Loncaster et al, 2001) and 81% of lung cancers (Swinson et al, 2003). However, in oesophageal cancer, many indicators of poor prognosis, such as Ki-67 and PCNA, which are cell proliferation markers, are expressed at the invasive front of the tumour. In this study, we found no significant correlation between CA9 expression at the tumour surface and invasive front and clinicopathological findings and prognosis (data not shown). In terms of IHC score, CA9 expression was significantly associated with tumour staging and poor prognosis. In other solid tumours, CA9 protein has been reported to be an independent factor related to poor prognosis (Chia et al, 2001; Loncaster et al, 2001; Brennan et al, 2006; Haapasalo et al, 2006; Lee et al, 2007). Driessen et al reported that CA9 expression was an independent prognostic marker in oesophageal cancers, especially adenocarcinomas and gastric cancer (Driessen et al, 2006), whereas in another study of oesophageal tumours, the degree of CA9 staining did not correlate with any of the pathological features examined (Turner et al, 1997). In our present study of ESCC, expression of CA9 protein was not independently related to poor prognosis. CA9 is a marker of hypoxia, and its expression is significantly correlated with tumour size. As a tumour grows in size, its centre gradually becomes starved of oxygen, and high expression of CA9 might therefore indicate that a tumour has a greater propensity for enlargement.
We studied the expression of CA9 mRNA in both normal and tumour tissues from 35 patients, using real-time RT–PCR. At the mRNA level, there was no significant correlation between CA9 expression and clinicopathological findings and prognosis (data not shown). In non-small-cell lung cancer, increased expression of CA9 mRNA in cancer tissues was significantly correlated with increased expression of the protein (Simi et al, 2006). However, in our study, we found no such correlation, perhaps because tissue samples were collected from the peripheral tumour areas, rather from the centre.
We confirmed in vitro that CA9 mRNA was expressed under hypoxic conditions in three ESCC cell lines, TE-2, TE-8 and TE-15, suggesting that upregulation of CA9 was controlled at the mRNA level. We also confirmed that CA9 was expressed at the protein level in these three ESCC cell lines under hypoxic conditions. Similar results have been reported for meningiomas (Yoo et al, 2007) and for several cell lines (Swinson et al, 2003; Robertson et al, 2004; Le et al, 2005). Comparison of the ESCC cell line TE-2 with an immortalised oesophageal cell line, CHEK-1, showed that expression of CA9 mRNA was induced in both under hypoxic conditions; however, the degree of CA9 expression differed greatly between the protein and the mRNA. This result suggests that CA9 might be induced in cells with higher malignant potential.
In this study, we also confirmed the presence of CA9 protein in the cell culture medium, a finding that has not been reported earlier. In ESCC cell lines, the level of CA9 in the culture medium was significantly increased under hypoxic conditions. CA9 is a membrane protein, and so it was very interesting to see that CA9 was present within the culture medium. It is unclear whether CA9 was secreted by the cancer cells or whether it was released from damaged cancer cells. In addition, we confirmed that oesophageal cancer cells under hypoxic conditions proliferated faster than cells under normoxic conditions, suggesting that CA9 has a role other than pH regulation alone. Robertson et al (2004) described that cells treated with CA9 interfering showed delayed growth under hypoxic conditions. In our study, cell proliferation and CA9 expression were induced under hypoxic conditions, suggesting that CA9 expression was correlated with cell proliferation.
In conclusion, our study has shown that hypoxia induces CA9 expression in ESCC and that this is related to poor prognosis. It has already been reported that (i) hypoxic conditions associated with tumours are related to resistance to chemotherapy and radiotherapy (Gray et al, 1953; Brizel et al, 1996; Hockel et al, 1996); (ii) low extracellular pH is a typical feature of the tumour microenvironment, which has an impact on cancer development (Svastová et al, 2004); and (iii) CA9 contributes to tumour growth and survival (Robertson et al, 2004). In addition, some ESCCs are relatively sensitive to chemotherapy and radiotherapy. Therefore, to improve the results of cancer therapy, it is important to control CA9, which is induced under hypoxic conditions and acidifies the tumour milieu. It is suggested that inhibition of CA9 may improve the effectiveness of chemotherapy and radiotherapy.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, Makuuchi H, Tanaka O, Yamana H, Ikeuchi S, Kabuto T, Nagai K, Shimada Y, Kinjo Y, Fukuda H, Japan Clinical Oncology Group (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol 21 (24): 4592–4596
Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, Rydén L, Gallagher WM, O’Brien SL (2006) CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 12 (21): 6421–6431
Brizel DM, Dodge RK, Clough RW, Dewhirst MW (1999) Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 53 (2): 113–117
Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56 (5): 941–943
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9 (2): 802–811
Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL (2001) Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol 19 (16): 3660–3668
Collard JM, Otte JB, Fiasse R, Laterre PF, De Kock M, Longueville J, Glineur D, Romagnoli R, Reynaert M, Kestens PJ (2001) Skeletonizing en bloc esophagectomy for cancer. Ann Surg 234 (1): 25–32
Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, Nafteux P, Penninckx F, Geboes K, Lerut T, Ectors N (2006) Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg 243 (3): 334–340
Fukai Y, Masuda N, Kato H, Fukuchi M, Miyazaki T, Nakajima M, Sohda M, Kuwano H, Nakajima T (2005) Correlation between laminin-5 gamma2 chain and epidermal growth factor receptor expression in esophageal squamous cell carcinomas. Oncology 69 (1): 71–80
Furuya M, Nishiyama M, Kimura S, Suyama T, Naya Y, Ito H, Nikaido T, Ishikura H (2004) Expression of regulator of G protein signalling protein 5 (RGS5) in the tumour vasculature of human renal cell carcinoma. J Pathol 203 (1): 551–558
Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61 (21): 7992–7998
Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26 (312): 638–648
Haapasalo JA, Nordfors KM, Hilvo M, Rantala IJ, Soini Y, Parkkila AK, Pastoreková S, Pastorek J, Parkkila SM, Haapasalo HK (2006) Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res 12 (2): 473–477
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56 (19): 4509–4515
Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI (1998) Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA 95 (21): 12596–12601
Kato H, Yoshikawa M, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Fukuchi M, Manda R, Tsukada K, Kuwano H (2002) Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) in esophageal squamous cell carcinoma. Anticancer Res 22 (6C): 3977–3984
Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos K, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001) Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res 7 (11): 3399–3403
Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O′Byrne KJ, Giaccia AJ, Koong AC (2005) Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol 23 (35): 8932–8941
Lee S, Shin HJ, Han IO, Hong EK, Park SY, Roh JW, Shin KH, Kim TH, Kim JY (2007) Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer Sci 98 (3): 329–333
Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM (2001) Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61 (17): 6394–6399
Maren TH (1967) Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev 47 (4): 595–781
Nishihira T, Hashimoto Y, Katayama M, Mori S, Kuroki T (1993) Molecular and cellular features of esophageal cancer cells. J Cancer Res Clin Oncol 119 (8): 441–449
Nordsmark M, Loncaster J, Aquino-Parsons C, Chou SC, Ladekarl M, Havsteen H, Lindegaard JC, Davidson SE, Varia M, West C, Hunter R, Overgaard J, Raleigh JA (2003) Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas. Radiother Oncol 67 (1): 35–44
Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA (2003) Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res 9 (13): 4641–4652
Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS (2000) Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA 97 (5): 2220–2224
Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, Zat’ovicová M, Liao S, Portetelle D, Stanbridge EJ, Závada J, Burny A, Kettmann R (1994) Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 9 (10): 2877–2888
Pastorekova S, Parkkila S, Pastorek J, Supuran CT (2004) Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 19 (3): 199–229
Robertson N, Potter C, Harris AL (2004) Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 64 (17): 6160–6165
Sashiyama H, Shino Y, Sakao S, Shimada H, Kobayashi S, Ochiai T, Shirasawa H (2002) Alteration of integrin expression relates to malignant progression of human papillomavirus-immortalized esophageal keratinocytes. Cancer Lett 177 (1): 21–28
Simi L, Venturini G, Malentacchi F, Gelmini S, Andreani M, Janni A, Pastorekova S, Supuran CT, Pazzagli M, Orlando C (2006) Quantitative analysis of carbonic anhydrase IX mRNA in human non-small cell lung cancer. Lung Cancer 52 (1): 59–66
Sobin LH, Witteking CH (eds) (2002) TNM Classificaton of Malignant Tumours, 6th edn. John Wiley & Sons, Inc: New York
Svastová E, Hulíková A, Rafajová M, Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastoreková S (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 577 (3): 439–445
Swinson DE, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O’Byrne KJ (2003) Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 21 (3): 473–482
Tashian RE (1989) The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays 10 (6): 186–192
Türeci O, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (1998) Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA 95 (13): 7608–7613
Turner JR, Odze RD, Crum CP, Resnick MB (1997) MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol 28 (6): 740–744
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60 (24): 7075–7083
Yoo H, Baia GS, Smith JS, McDermott MW, Bollen AW, Vandenberg SR, Lamborn KR, Lal A (2007) Expression of the hypoxia marker carbonic anhydrase 9 is associated with anaplastic phenotypes in meningiomas. Clin Cancer Res 13 (1): 68–75
Acknowledgements
We thank T Yajima, S Tsutsumi, E Yamaki and K Araki for helpful discussions. We are also grateful to R Aoyagi for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tanaka, N., Kato, H., Inose, T. et al. Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer 99, 1468–1475 (2008). https://doi.org/10.1038/sj.bjc.6604719
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604719
Keywords
This article is cited by
-
Characterization of residual cancer by comparison of a pair of organoids established from a patient with esophageal squamous cell carcinoma before and after neoadjuvant chemotherapy
Human Cell (2024)
-
JmjC-KDMs KDM3A and KDM6B modulate radioresistance under hypoxic conditions in esophageal squamous cell carcinoma
Cell Death & Disease (2020)
-
Influence of hypoxia-dependent factors on the progression of neuroblastoma
Pediatric Surgery International (2016)
-
High expression of carbonic anhydrase IX is significantly associated with glandular lesions in gastroesophageal junction and with tumorigenesis markers BMI1, MCM4 and MCM7
BMC Gastroenterology (2015)
-
Biodistribution and Radiation Dosimetry of the Carbonic Anhydrase IX Imaging Agent [18 F]VM4-037 Determined from PET/CT Scans in Healthy Volunteers
Molecular Imaging and Biology (2014)