Abstract
The literature reports low rates of breast conservation after neoadjuvant chemotherapy for operable breast cancers not amenable to initial breast-conserving surgery. This study aims to compare the outcome of lobular vs ductal carcinomas after neoadjuvant chemotherapy. Between 1989 and 1999, 750 patients with clinical stage II/IIIA ductal (672) or lobular (78) invasive breast carcinomas were treated at the Institut Curie with primary anthracycline-based polychemotherapy followed by either breast conservation (surgery and/or radiotherapy) or mastectomy. Median follow-up was 10 years. Clinical response to primary chemotherapy was significantly worse for lobular than for ductal carcinomas (47 vs 60%; P=0.04), but only histological grade remained predictive in multivariate analysis. Breast conservation was high for both ductal and lobular carcinomas (65 and 54%; P=0.07), due, in part, to the use of radiotherapy, either exclusive or preoperative, for respectively 26 and 40% of patients. The lobular type had no adverse effect, neither on locoregional control nor on overall survival, even in the group of patients treated with breast conservation.
Similar content being viewed by others
Main
The standard treatment of operable breast adenocarcinomas too large to be amenable to breast-conserving surgery is mastectomy with axillary lymph node clearance. Several randomised studies have compared neoadjuvant with adjuvant chemotherapy. In these studies, the locoregional treatment could be either surgery or radiotherapy or a combination of both. There was no significant difference in terms of locoregional control and overall (OS) or disease-free survivals (Mauri et al, 2005). The principal clinical benefit of a neoadjuvant medical treatment was, in the case of good clinical response, avoiding mastectomy. However, data regarding the specific behaviour of invasive lobular carcinomas (ILC), a histological subtype of breast cancers representing 5–15% of all breast cancer cases, are still scarce in this therapeutic setting. Lobular carcinomas are characterised by a specific morphology with discohesive small cells usually associated with estradiol receptor (ER), progesterone receptor (PR) positivity and with a low proliferation rate (Sastre-Garau et al, 1996). These factors are now well-established predictive markers of poor response to neoadjuvant chemotherapy (Pierga et al, 2003). We, and others, have previously shown that ILC are often diagnosed as larger tumours than invasive ductal carcinomas (IDC) (Sastre-Garau et al, 1996), and for which a neoadjuvant treatment could be chosen to increase the rate of conservative surgery. This study aims to compare the outcome of lobular vs ductal carcinomas under neoadjuvant chemotherapy and to report their locoregional control.
Materials and methods
Between January 1989 and December 1999, a total of 803 patients, with operable, clinical stage II/IIIA, either ductal or lobular invasive breast carcinoma, not amenable to initial breast-conserving surgery, were treated at the Institut Curie with primary chemotherapy. Out of them, 750 had an initial core-needle biopsy before the start of treatment and will be studied here – 78 patients with lobular invasive carcinoma and 672 with ductal invasive carcinomas. The other 53 had initial fine-needle aspiration and underwent biopsy after the start of the chemotherapy. Median follow-up was 117 months (10–188). Histological classification was made according to the WHO criteria. Histological grading was performed according to the Scarff Bloom and Richardson method (Bloom and Richardson, 1957). Positivity to ER and PR was determined by biochemistry. Hormonal receptors (HR) were considered positive when either ER or PR was positive. All slides of lobular carcinomas were retrospectively reviewed. The E-cadherin expression was assessed by immunohistochemistry.
Treatment decisions were not adapted to the histological type. The following information regarding the treatment details apply both to lobular and ductal carcinomas. No patient received neoadjuvant hormonal therapy. All patients received a median of four (range: 1–6) cycles of neoadjuvant chemotherapy. Chemotherapy between 1989 and 1995, consisted of FAC with 5-Fluorouracil (F) on days 1, 3, 5 and 8, adriamycin (A) on days 1 and 8, and cyclophosphamide (C) on days 1 and 8 (Pierga et al, 2003). It was subsequently simplified according to a more classical regimen with adriamycin or epirubicin (E), cyclophosphamide and/or fluorouracil every 21 days for six cycles (FAC, FEC, AC or EC) (French Adjuvant Study Group, 2001).
Response to primary chemotherapy was assessed clinically as reported by Pierga et al (2003), according to the International Union against Cancer Criteria. Complete response was defined as the total resolution of the breast mass and regional lymph adenopathy as determined by physical examination; partial response was defined as 50% or greater reduction in the product of the two largest perpendicular dimensions of the breast mass and regional adenopathy; minor response was defined as less than a 50% reduction in the product of the two largest perpendicular dimensions. Stable disease was defined as no measurable change in the product of the two largest perpendicular dimensions, and progressive disease was defined as an increase of at least 25% in the product of the two largest perpendicular dimensions. Clinical response was dichotomised in two groups: <50 and ⩾50%.
Local treatments consisted in breast surgery and/or radiotherapy. Whenever feasible, it consisted in tumorectomy followed by radiotherapy. When the tumour did not become amenable to conservative surgery, the decision was usually made to perform a mastectomy, followed in most cases by radiotherapy. However, some patients, because of a desire to conserve their breasts, received radiotherapy as first (followed by either tumorectomy or mastectomy) or exclusive local treatment. In other instances, this sequence of preoperative or exclusive radiotherapy was chosen for tumours with very good response to chemotherapy. Radiotherapy delivered a mean dose of 51.4 Gy (s.d. 2.5 Gy) in 2 Gy fractions to the breast, using either standard or lateral decubitus techniques (Campana et al, 2005), or to the chest wall. A mean dose of 45 Gy (s.d., 2 Gy) in 23–25 fractions was usually delivered to the internal mammary chain and the supraclavicular area with the addition of an axillary irradiation when there was an important axillary involvement or in the absence of an axillary lymph node dissection. In the case of breast–conserving treatments, a boost was delivered to the tumour or to the tumorectomy bed by either external beam radiotherapy or brachytherapy. The mean total dose was 64 Gy (s.d., 7.6 Gy) to the tumorectomy bed for postoperative radiotherapy and 73 Gy (s.d., 7.6 Gy) to the tumour in the case of exclusive radiotherapy. Patients were followed up clinically every 6 months for the first 5 years and annually thereafter. Mammograms and/or ultrasound scans were performed annually.
Statistics
All studied factors are reported in the relevant Tables.
Differences between groups were analysed by χ2 tests for categorical variables and Student's t-test for continuous variables.
Univariate logistic regressions were performed to identify predictive factors of clinical response (⩾50%) and to estimate crude odds ratios (OR). Multivariate logistic regression analyses permitted to estimate the OR of variables independently associated with clinical response.
Survival data were defined as the time from diagnosis of breast cancer until the occurrence of event, that is, locoregional recurrence (LRR) defined as any recurrence occurring in the treated breast or in the ipsilateral chest wall or regional lymph node-bearing areas (internal mammary chain, axilla and supraclavicular area) and death (OS). Recurrence-free and alive patients were censored at the date of their last known contact. The OS and LRR rates were calculated by the Kaplan–Meier method, and groups were compared using a log-rank test. Multivariate analysis was carried out to assess the relative influence of prognostic factors on locoregional control, using the Cox stepwise procedure after stratification on the local treatment (exclusive/preoperative radiotherapy vs postoperative radiotherapy) (Cox and Oakes, 1984). Missing values (tumour grade, receptor levels and mitotic index) were coded as separate categories of the variable and retained in the model.
Significance level was 0.05. Analyses were performed using Splus 2000 software (Mathsoft Inc., Seattle, WA, USA).
Results
Patients' and tumours' characteristics according to the histological type of invasive carcinoma
The comparison of the 78 patients with lobular invasive carcinoma with the 672 with ductal invasive carcinomas is summarised in Table 1.
Patients diagnosed with lobular invasive carcinomas were significantly older than patients with ductal carcinomas. Lobular cancers were significantly larger (median size of 50 vs 40 mm; P<10−3) with more HRs positive (HR+; 87 vs 73%; P=0.02) and lower histological grades than ductal carcinomas (grade 1; 41 vs 12%; P<10−3).
Response to primary chemotherapy and local treatment according to histological type
Clinical response to primary chemotherapy was significantly better for ductal invasive carcinomas than for lobular (⩾50% of clinical response in 60% for ductal vs 47% for lobular; P=0.04; Table 2). The rates of breast-conserving treatments for lobular carcinomas was lower than that for ductal carcinomas, but the difference was not statistically significant (54% (42 out of 78) vs 65% (434 out of 672); P=0.07) and more patients with lobular carcinomas received local treatment with either exclusive radiotherapy or preoperative radiotherapy compared with ductal carcinomas (40 (31 out of 78) vs 26% (178 out of 672); P=0.02). If we look at patients who had surgery, excluding patients with radiotherapy alone as local treatment, there were significantly fewer breast conservations for lobular than for ductal invasive carcinomas: 24 out of 78 (31%) vs 333 out of 672 (50%), P=0.001. Rates of pathological complete response (for all patients who had undergone surgery) were respectively 9% (40 out of 457, 118 unknown) for ductal and 8% (4 out of 50, 10 unknown) for lobular (P=0.94).
Other factors of good clinical response found in univariate analysis (Table 3) were clinical T2 vs T3, HR+ vs HR− and high histological grade vs low and intermediate. In multivariate analysis, only high grade was significantly associated with a higher rate of clinical response.
Comparison of patients who underwent breast conservation with either preoperative/exclusive radiotherapy or postoperative radiotherapy
Among patients who received breast-conserving treatments, those with preoperative/exclusive radiotherapy had tumours that were significantly more often clinically T3 (23 vs 15%; P=0.03), lobular (15 vs 5%; P<10−3) and responded clinically less to primary chemotherapy (clinical response ⩾50% respectively 63 vs 78%; P<10−3) than those with postoperative radiotherapy (Table 4).
Survivals
Overall survival rates at, respectively, 5 and 10 years were 81 (95% CI, 78–84%) and 66% (95% CI, 62–70%).
Locoregional control rates at, respectively, 5 and 10 years were 83 (95% CI, 81–86%) and 75% (95% CI, 71–78%) but, when considering only patients treated with breast conservation, 81 (95% CI, 77–85%) and 71% (95% CI, 67–76%).
Prognostic factors
For locoregional control
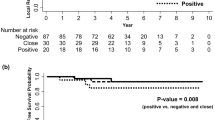
In univariate analysis, factors that were found to be associated with a higher locoregional recurrence rate were young age, cN1, HR− and having undergone a breast-conserving treatment (Table 5). The histological subtype classification (lobular vs ductal) was not found to be prognostic for locoregional control, with values at 10 years being 73 and 75%, respectively (RR=1 (0.6–1.7); P=0.9).
On forward stepwise multivariate analysis (Cox model) stratified on local treatment, only the young age (<40 years) remained statistically significant.
When considering only patients treated with breast conservation (Table 6), the only factors found in univariate analysis to be associated with a higher locoregional recurrence rate were young age and treatment with preoperative or exclusive radiotherapy. The histological subtype classification (lobular vs ductal) was not found to be prognostic for locoregional control with, respectively, at 10 years, a locoregional control of 62 and 72% (RR=1.5 (0.9–2.6); P=0.12).
On multivariate analysis (Cox model) stratified on local treatment, only young age (<40 years) remained statistically associated with worse locoregional control.
For overall survival
In univariate analysis, factors that were found to be associated with a lower overall survival rate were cT3, cN1, HR−, histological grade 2 and 3 and mitotic index grade 2 and 3 (Table 7). The histological subtype classification (lobular vs ductal) was not found to be prognostic for overall survival with, respectively, at 10 years, a rate of 72% (95% CI, 62–84%) and 65% (95% CI, 61–69); RR=0.84 (0.55–1.30); P=0.44.
On forward stepwise multivariate analysis (Cox model) stratified on local treatment, cT3, cN1, HR−, histological grade 2/3 and absence of clinical response were statistically significant.
Discussion
This retrospective study spans a period of 10 years with patients treated between 1989 and 1999. The follow-up is long with a median at 10 years and makes the study of locoregional survival relevant. The tumour characteristics of lobular carcinomas were those already found by Sastre and co-workers and others (Sastre-Garau et al, 1996; Cristofanilli et al, 2005). Patients diagnosed with lobular invasive carcinomas were significantly older (Sastre-Garau et al, 1996) than patients with ductal tumours. Lobular cancers were significantly larger (Sastre-Garau et al, 1996; Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006) with more HRs (Sastre-Garau et al, 1996; Mathieu et al, 2004; Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006) and lower histological (Sastre-Garau et al, 1996; Tubiana-Hulin et al, 2006) or nuclear (Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006) grade than ductal carcinomas.
Because of the frequent use of either exclusive or preoperative radiotherapy, the study of pathological response in our series was not relevant. Bearing in mind the mandatory caution in interpreting clinical response (Bollet et al, 2007), we found that the response to primary chemotherapy was significantly better for ductal invasive carcinomas than for lobular (⩾50% of clinical response in 60% for ductal vs 47% for lobular; P=0.04), confirming the notion developed by others that lobular carcinomas responded less than ductal carcinomas to neoadjuvant chemotherapy (Mathieu et al, 2004; Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006; Wenzel et al, 2006; Katz et al, 2007). However, in the present study, in multivariate analysis, only high grade was significantly associated with a higher rate of clinical response. This result is therefore in agreement with the Nomograms's design proposed online, which does not take into account the histological type of the tumour when deciding on the therapeutic option (Rouzier et al, 2005b). There is room to better define biological predictive factors of response to neoadjuvant chemotherapy to screen patients who would benefit most from this therapeutic option (Sotiriou et al, 2002; Chang et al, 2005; Gianni et al, 2005; Rouzier et al, 2005a; Pierga et al, 2007). Lobular carcinomas had reduced clinical responses to neoadjuvant chemotherapy, while responses to breast-conserving treatments were fewer, although not significantly (54% (42 out of 78) vs 65% (434 out of 672); P=0.07). The lower rate of breast conservation in lobular vs ductal invasive carcinoma could also be due to the larger size of ILC. The most striking data are the high rate of breast conservation for both ductal and even more so for lobular carcinomas when compared to the rates in the literature that range, respectively, between 30–48 and 16–31% (Mathieu et al, 2004; Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006). This is explained in our series by the use of either exclusive or preoperative radiotherapy, particularly for large tumours with a poor response to neoadjuvant chemotherapy and, therefore, more frequently for lobular (40%) than for ductal (26%) carcinomas. Because of the retrospective nature of this study, we could not report the reason for the choice of performing preoperative or exclusive radiotherapy. However, as one can see from Table 2, the proportion of patients who received radiotherapy immediately after primary chemotherapy and who actually went on to have successful breast-conserving treatments was 80 and 84%, respectively, for IDC and ILC.
Despite being associated with large tumour size and less clinical response to primary chemotherapy, lobular subtype was not found associated with either worse locoregional control, even in the subgroup of only patients who had been treated with breast conservation, or worse overall survival. It should, however, be noted that in this series, patients treated with exclusive or preoperative radiotherapy fared worse in terms of locoregional control than others, perhaps also because of a selection of patients with poor prognosis. This opens the debate as to whether radiotherapy could not play a role in increasing the rate of breast-conserving treatments for some invasive carcinomas, as it is an important part of the treatment of large breast cancers (Whelan et al, 2000; Clarke et al, 2005) and has been shown to achieve complete clinical response (6–41%) with doses compatible with planned secondary surgeries (Calitchi et al, 1991; Scholl et al, 1994; Broet et al, 1999; Bollet et al, 2006). At the Institut Curie, the S6 trial randomised premenopausal women between chemotherapy and radiotherapy as an initial treatment for large breast cancers (Scholl et al, 1994). There was no difference in the 10-year OS (Broet et al, 1999). Out of 69 tumours still palpable after four cycles of FAC (5-fluorouracil, adriamycine and cyclophosphamide), 42 (61%) achieved complete clinical response after 54 Gy. In contrast with chemotherapy (Vincent-Salomon et al, 2004; Andre et al, 2005), which is more effective in proliferative tumours, the effect of radiotherapy does not seem to depend on the degree of proliferation (or on the histological grade) (Remvikos et al, 1993; Rozan et al, 1998; Clarke et al, 2006). Radiotherapy could thus theoretically play a role in increasing the rate of breast conservation for tumours that fail to sufficiently shrink with primary chemotherapy because of low proliferation, as in the case of lobular carcinomas. Another study performed at the Institut Curie showed a similar rate of pathological complete response with preoperative concurrent chemoradiotherapy for lobular and for ductal invasive carcinomas (31 vs 22%; P=0.5; Bollet et al, 2006). Ring et al (2003) found, in a retrospective study comparing surgery (either breast-conserving or mastectomy) and exclusive radiotherapy for women who achieved clinical complete responses after primary chemotherapy, a nonsignificant trend toward increased locoregional-only recurrence for the no surgery group. However, they also found that patients achieving both clinical and ultrasound complete responses, and not undergoing surgery, had a low (8%) rate of locoregional recurrence at 5 years. Additionally, MRI has also proven to be useful in the assessment of the tumour response to chemotherapy (Thibault et al, 2004; Warren et al, 2004; Partridge et al, 2005; Segara et al, 2007). This good value of MRI in the evaluation of tumour response should also apply to ILC as, even though ILC is still a challenge for MRI (Kinkel and Hylton, 2001), the addition of MRI to mammography still increases the sensitivity in the detection of ILC (Gilles et al, 1994; Sittek et al, 1998) and, most importantly in the case of the response evaluation, the volumetric assessment is improved (Boetes et al, 2004; Fabre Demard et al, 2005; Caramella et al, 2007; Mann et al, 2008). This raises the question of whether patients who achieve complete clinical and imaging response could in some cases entirely avoid surgery. This issue, needless to say, would need to be properly assessed in a prospective randomised trial. Additionally, to really benefit from higher rates of breast conservation, one needs to closely evaluate the cosmetic effects of this therapeutic approach but which could not be assessed in our study. One particular concern is indeed that it is associated with worse cosmetic results, because they deteriorate with the radiotherapy total dose (whole breast+boost; Vrieling et al, 1999), with chemotherapy (Vass and Bairati, 2005), and when radiotherapy is given pre- rather than post-operatively, both in the case of standard (Durand et al, 1987) or oncoplastic (Clough et al, 2003) surgeries.
In conclusion, our series confirmed that lobular carcinomas demonstrate a lower response rate to neoadjuvant chemotherapy than ductal carcinomas, but that in multivariate analysis only low histological grades remained predictive of poor response to chemotherapy. The breast conservation in our series was higher than the one expected from the literature for both histological types. The most obvious explanation was the frequent use of either preoperative or exclusive radiotherapy after chemotherapy, especially for tumours with a poor response to chemotherapy and therefore for lobular carcinomas. The lobular type had no adverse effect on locoregional control even in the group of patients treated with breast conservation. Further studies are still needed to evaluate the exact role of preoperative/exclusive radiotherapy after neoadjuvant chemotherapy, not only in terms of efficacy but also of tolerance and long-term cosmetic effects.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andre F, Khalil A, Slimane K, Massard C, Mathieu MC, Vignot S, Assi H, Delaloge S, Spielmann M (2005) Mitotic index and benefit of adjuvant anthracycline-based chemotherapy in patients with early breast cancer. J Clin Oncol 23: 2996–3000
Bloom HJ, Richardson WW (1957) Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 11: 359–377
Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO (2004) The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat 86: 31–37
Bollet MA, Sigal-Zafrani B, Gambotti L, Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Dieras V, Fourquet A, For The Institut Curie Breast Cancer Study G (2006) Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: results of a phase II study. Eur J Cancer 42: 2286–2295
Bollet MA, Thibault F, Bouillon K, Meunier M, Sigal-Zafrani B, Savignoni A, Dieras V, Nos C, Salmon R, Fourquet A, Institut Curie Breast Cancer Study Group (2007) Role of dynamic magnetic resonance imaging in the evaluation of tumor response to preoperative concurrent radiochemotherapy for large breast cancers: a prospective phase II study. Int J Radiat Oncol Biol Phys 69: 13–18
Broet P, Scholl SM, de la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y, Asselain B, Pouillart P (1999) Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat 58: 151–156
Calitchi E, Otmezguine Y, Feuilhade F, Piedbois P, Pavlovitch JM, Brun B, Mazeron JJ, Le Bourgeois JP, Julien M, Pierquin B (1991) External irradiation prior to conservative surgery for breast cancer treatment. Int J Radiat Oncol Biol Phys 21: 325–329
Campana F, Kirova YM, Rosenwald JC, Dendale R, Vilcoq JR, Dreyfus H, Fourquet A (2005) Breast radiotherapy in the lateral decubitus position: a technique to prevent lung and heart irradiation. Int J Radiat Oncol Biol Phys 61: 1348–1354
Caramella T, Chapellier C, Ettore F, Raoust I, Chamorey E, Balu-Maestro C (2007) Value of MRI in the surgical planning of invasive lobular breast carcinoma: a prospective and a retrospective study of 57 cases: comparison with physical examination, conventional imaging, and histology. Clin Imaging 31: 155–161
Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Tham YL, Kalidas M, Elledge R, Mohsin S, Osborne CK, Chamness GC, Allred DC, Lewis MT, Wong H, O'Connell P (2005) Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J Clin Oncol 23: 1169–1177
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366: 2087–2106
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2006) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366: 2087–2106
Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC (2003) Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 237: 26–34
Cox DR, Oakes D (1984) Analysis of Survival Data. London: Chapman & Hall
Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, Valero V, Buzdar AU, Kuerer H, Buccholz TA, Hortobagyi GN (2005) Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol 23: 41–48
Durand JC, Solalinde V, Mathieu G, Salmon RJ, Bataini P, Vilcoq JR, Hamelin JP, Pilleron JP (1987) Conservative surgery after preoperative radiotherapy in the treatment of breast cancer. Indications and results. Bull Cancer 74: 641–646
Fabre Demard N, Boulet P, Prat X, Charra L, Lesnik A, Taourel P (2005) Breast MRI in invasive lobular carcinoma: diagnosis and staging. J Radiol 86: 1027–1034
French Adjuvant Study Group (2001) Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French adjuvant study group 05 randomized trial. J Clin Oncol 19: 602–611
Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, Mariani G, Rodriguez J, Carcangiu M, Watson D, Valagussa P, Rouzier R, Symmans WF, Ross JS, Hortobagyi GN, Pusztai L, Shak S (2005) Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 23: 7265–7277
Gilles R, Guinebretiere JM, Lucidarme O, Cluzel P, Janaud G, Finet JF, Tardivon A, Masselot J, Vanel D (1994) Nonpalpable breast tumors: diagnosis with contrast-enhanced subtraction dynamic MR imaging. Radiology 191: 625–631
Katz A, Saad ED, Porter P, Pusztai L (2007) Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol 8: 55–62
Kinkel K, Hylton NM (2001) Challenges to interpretation of breast MRI. J Magn Reson Imaging 13: 821–829
Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C (2008) The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol 34: 135–142
Mathieu MC, Rouzier R, Llombart-Cussac A, Sideris L, Koscielny S, Travagli JP, Contesso G, Delaloge S, Spielmann M (2004) The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer 40: 342–351
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97: 188–194
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, Rugo HS, Hwang ES, Ewing CA, Hylton NM (2005) MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 184: 1774–1781
Pierga JY, Mouret E, Laurence V, Dieras V, Savigioni A, Beuzeboc P, Dorval T, Palangie T, Jouve M, Pouillart P (2003) Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. The role of clinical response. Eur J Cancer 39: 1089–1096
Pierga JY, Reis-Filho JS, Cleator SJ, Dexter T, Mackay A, Simpson P, Fenwick K, Iravani M, Salter J, Hills M, Jones C, Ashworth A, Smith IE, Powles T, Dowsett M (2007) Microarray-based comparative genomic hybridisation of breast cancer patients receiving neoadjuvant chemotherapy. Br J Cancer 96: 341–351
Remvikos Y, Mosseri V, Zajdela A, Fourquet A, Durand JC, Pouillart P, Magdelenat H (1993) Prognostic value of the S-phase fraction of breast cancers treated by primary radiotherapy or neoadjuvant chemotherapy. Ann NY Acad Sci 698: 193–203
Ring A, Webb A, Ashley S, Allum WH, Ebbs S, Gui G, Sacks NP, Walsh G, Smith IE (2003) Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer? J Clin Oncol 21: 4540–4545
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005a) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11: 5678–5685
Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, Symmans WF, Wagner P, Atallah D, Valero V, Berry DA, Hortobagyi GN (2005b) Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol 23: 8331–8339
Rozan S, Vincent-Salomon A, Zafrani B, Validire P, De Cremoux P, Bernoux A, Nieruchalski M, Fourquet A, Clough K, Dieras V, Pouillart P, Sastre-Garau X (1998) No significant predictive value of c-erbB-2 or p53 expression regarding sensitivity to primary chemotherapy or radiotherapy in breast cancer. Int J Cancer 79: 27–33
Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, Durand JC, Fourquet A, Pouillart P (1996) Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 77: 113–120
Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangie T, Jouve M, Beuzeboc P, Garcio-Giralt E, Salmon RJ, de la Rochefordiere A, Campana F, Pouillart P (1994) Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 30A: 645–652
Segara D, Krop IE, Garber JE, Winer E, Harris L, Bellon JR, Birdwell R, Lester S, Lipsitz S, Iglehart JD, Golshan M (2007) Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol 96: 474–480
Sittek H, Perlet C, Untch M, Kessler M, Reiser M (1998) Dynamic MR-mammography in invasive lobular breast cancer. Rontgenpraxis 51: 235–242
Sotiriou C, Powles TJ, Dowsett M, Jazaeri AA, Feldman AL, Assersohn L, Gadisetti C, Libutti SK, Liu ET (2002) Gene expression profiles derived from fine needle aspiration correlate with response to systemic chemotherapy in breast cancer. Breast Cancer Res 4: R3
Thibault F, Nos C, Meunier M, El Khoury C, Ollivier L, Sigal-Zafrani B, Clough K (2004) MRI for surgical planning in patients with breast cancer who undergo preoperative chemotherapy. AJR Am J Roentgenol 183: 1159–1168
Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiere JM, Bouita L, Cohen-Solal C, Cherel P, Rouesse J (2006) Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol 17: 1228–1233
Vass S, Bairati I (2005) A cosmetic evaluation of breast cancer treatment: a randomized study of radiotherapy boost technique. Int J Radiat Oncol Biol Phys 62: 1274–1282
Vincent-Salomon A, Rousseau A, Jouve M, Beuzeboc P, Sigal-Zafrani B, Freneaux P, Rosty C, Nos C, Campana F, Klijanienko J, Al Ghuzlan A, Sastre-Garau X (2004) Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline-based preoperative chemotherapy. Eur J Cancer 40: 1502–1508
Vrieling C, Collette L, Fourquet A, Hoogenraad WJ, Horiot JC, Jager JJ, Pierart M, Poortmans PM, Struikmans H, Van der Hulst M, Van der Schueren E, Bartelink H (1999) The influence of the boost in breast-conserving therapy on cosmetic outcome in the EORTC ‘boost versus no boost’ trial. EORTC radiotherapy and breast cancer cooperative groups. European organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys 45: 677–685
Warren RM, Bobrow LG, Earl HM, Britton PD, Gopalan D, Purushotham AD, Wishart GC, Benson JR, Hollingworth W (2004) Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy? Br J Cancer 90: 1349–1360
Wenzel C, Bartsch R, Hussian D, Pluschnig U, Altorjai G, Zielinski CC, Lang A, Haid A, Jakesz R, Gnant M, Steger GG (2006) Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel+G-CSF. Breast Cancer Res Treat 104: 109–114
Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML (2000) Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 18: 1220–1229
Acknowledgements
We thank all the members of the Breast Cancer Study Group at the Institut Curie who have contributed to the completion of this study. We also thank Chantal Gautier for her help in the management of the patients' data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Bollet, M., Savignoni, A., Pierga, JY. et al. High rates of breast conservation for large ductal and lobular invasive carcinomas combining multimodality strategies. Br J Cancer 98, 734–741 (2008). https://doi.org/10.1038/sj.bjc.6604229
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604229
Keywords
This article is cited by
-
Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma
Modern Pathology (2020)
-
Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer
Breast Cancer Research and Treatment (2013)
-
Les traitements néoadjuvants (hors cancer du sein inflammatoire)
Oncologie (2011)
-
Surgical Considerations Following Preoperative Systemic Chemotherapy
Current Breast Cancer Reports (2011)