Abstract
An immunohistochemical study was performed using tissue microarrays and specific antibodies against matrix metalloproteinases (MMPs) 1, 2, 7, 9, 11, 13, 14, and their tisullar inhibitors (TIMPs) 1, 2, and 3. More than 2600 determinations on cancer specimens from 131 patients with primary ductal invasive tumours of the breast (65 with and 66 without distant metastasis) and controls were performed. Staining results were categorised using a score based on the intensity of the staining and a specific software program calculated the percentage of immunostained cells automatically. We observed a broad variation of the total immunostaining scores and the cell type expressing each protein. There were multiple and significant associations between the expression of the different MMPs and TIMPs evaluated and some parameters indicative of tumour aggressiveness, such as large tumour size, advanced tumour grade, high Nottinham prognostic index, negative oestrogen receptor status, peritumoural inflammation, desmoplastic reaction, and infiltrating tumoural edge. Likewise, the detection of elevated immunohistochemical scores for MMP-9, 11, TIMP-1, and TIMP-2, was significantly associated with a higher rate of distant metastases. The expression of MMP-9 or TIMP-2 by tumour cells, MMP-1, 7, 9, 11, 13, or TIMP-3 by fibroblastic cells, and MMP-7, 9, 11, 13, 14, TIMP-1, or TIMP-2 by mononuclear inflammatory cells, was also significantly associated with a higher rate of distant metastases.
Similar content being viewed by others
Main
Relapse in the form of metastases within 5 years of surgery occurs in about half the women with primary breast cancer with originally apparently localised tumours. However, it is difficult to predict this event because breast cancer is a heterogeneous disease encompassing a variety of pathological entities and a wide range of clinical behaviours, even in patient groups that appear to be clinically similar. Therefore, and despite having a number of classical prognostic variables available, new prognostic factors should be identified to improve the present risk classification and thereby to develop a more rational management of breast cancer patients.
Tumour invasion and metastasis development are the primary determinants of patient outcome and, accordingly, molecules involved in these processes are obvious candidates to be identified as new prognostic markers in breast cancer. Degradation of the stromal connective tissue and basement membrane components are key elements in tumour invasion and metastasis. Proteolytic enzymes of various classes execute the breaking down of matrix elements. However, some components, particularly the interstitial collagens, are very resistant to proteolytic attacks, being degraded only by matrix metalloproteinases (MMPs) (Nelson et al, 2000). The human MMP family currently consists of 28 members of homologous zinc-dependent endopeptidases that can be divided into eight structural classes or, on the basis of their substrate specificity and primary structure, into the more familiar subgroups of collagenases (MMP-1, 8, and 13), gelatinases (MMP-2 and 9), stromelysins (MMP-3, 10, and 11), membrane-associated MMPs (MMP-14, 15, 16, 17, 23, 24, and 25), and other novel MMPs (Brinckerhoff et al, 2000; Overall and Lopez-Otin, 2002; Demers et al, 2005). Matrix metalloproteinases are synthesised as inactive zymogens, which are then activated predominantly pericellularly either by other MMPs or by serine proteases. The activity of MMPs is specifically inhibited by the so-called tissue inhibitors of metalloproteases (tisullar inhibitors (TIMPs)). Currently, four different TIMPs are known to exist: TIMPs 1, 2, 3, and 4.
There are available data clearly challenging the classic dogma stating that MMPs promote metastases exclusively by modulating the remodelling of extracellular matrix, as MMPs able to impact in vivo on tumour cell behaviour as a consequence of their ability to cleave growth factors, cell surface receptors, cell adhesion molecules, or chemokines/cytoquines have also been identified (Manes et al, 1999; Noe et al, 2001; Egeblad and Werb, 2002; Turk et al, 2004). Furthermore, by cleaving proapoptotic factors, MMPs are able to produce a more aggressive phenotype via generation of apoptotic resistant cells (Fingleton et al, 2001). Matrix metalloproteinases may also regulate cancer/related angiogenesis, both positively through their ability to mobilise or activate proangiogenic factors (Stetler-Stevenson, 1999) and negatively via generation of angiogenesis inhibitors, such as angiostatin and endostatin, cleaved from large protein precursors (Cornelius et al, 1998). In addition, it is now assumed that TIMPs are multifactorial proteins also involved in the induction of proliferation and the inhibition of apoptosis (Jiang et al, 2002; Wurtz et al, 2005).
The objectives of the present work were to evaluate the morphological expression and clinical relevance of several MMPs and TIMPs of biological importance in invasive ductal carcinomas of the breast, by using the tissue microarray (TMA) technique, which has allowed us to process a large number of tissue specimens for a wide range of protein determinations (Kononen et al, 1998; Camp et al, 2000).
Materials and methods
Patients' selection, patients' characteristics, and tissue specimen handling
This study comprised 131 women with a histologically confirmed diagnosis of early breast cancer and treated between 1990 and 2001. We selected women with the following inclusion criteria: invasive ductal carcinoma, at least 10 histopathologicallyassessed axillary lymph nodes, and a minimum of 5 years of follow-up in those women without tumoral recurrence. The exclusion criteria were the following: metastatic disease at presentation, prior history of any type of malignant tumour, bilateral breast cancer at presentation, having received any type of neoadjuvant therapy, development of loco-regional recurrence during the follow-up period, development of a second primary cancer, and absence of sufficient tissue in the paraffin blocks used for manufacturing the TMAs. From a total of 1053 patients fulfilling these criteria, we selected randomly a sample size of 131 patients, in accordance with four different groups of similar size and stratified with regard to nodal status and with the development of metastatic disease, which were the key measure variables of the study. Thus, we include an important number of events in both node-negative and node-negative patient subgroups (half the cases with distant metastasis during the follow-up period in each one of these subgroups) for securing the statistical power of the survival analysis. Patients' characteristics included in the two main groups, with or without distant metastases, are listed in Table 1. Nottingham prognostic grade was assessed in accordance with Galea (1992).
Women were treated according to the guidelines used in our institution. The study adhered to national regulations and was approved by our institution's Ethics and Investigation Committee. The end point was distant metastatic relapse. The median follow-up period in patients without metastasis was 87.5 months, and 52.7 months in patients with metastasis.
Tissue microarrays and immunohistochemistry
Routinely fixed (overnight in 10% buffered formalin), paraffin-embedded tumour samples stored in our pathology laboratory files were used in this study. Histopathologically representative tumour areas were defined on haematoxylin and eosin (H&E)-stained sections and marked on the slide. Tumour tissue array blocks were obtained by punching a tissue cylinder (core) with a diameter of 1.5 mm through a histologically representative area of each ‘donor’ tumour block, which was then inserted into an empty ‘recipient’ tissue array paraffin block using a manual tissue arrayer (Beecker Instruments, Sun Praerie, Winconsin, USA) as described elsewhere (Parker et al, 2002). Collection of tissue cores was carried out under highly controlled conditions. Areas of non-necrotic cancerous tissue were selected for arraying by two experienced pathologists (LO González and AM Merino). Two cores were employed for each case. From the 131 tumour samples available, four tissue array blocks were prepared, each containing 33 tumour samples, as well as internal controls including four normal breast tissue samples from two healthy women that underwent reductive mammary surgery.
Four composite high-density TMA blocks were designed, and serial 5 μm sections were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slides. One section from each tissue array block was stained with H&E, and these slides were then reviewed to confirm that the sample was representative of the original tumour. Immunohistochemistry was carried out on these sections of TMA fixed in 10% buffered formalin and embedded in paraffin using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Antibodies for MMPs and TIMPs were obtained from Neomarker (Lab Vision Corporation, Fremont, CA, USA). The dilution for each antibody was established based on negative and positive controls (1/50 for MMP-2, 7, and 14, TIMP-2 and 3; 1/100 for MMP-1, 9, and 13 and TIMP-1; and 1/200 for MMP-11).
Tissue sections were deparaffinised in xylene, and then rehydrated in graded concentrations of ethyl alcohol (100, 96, 80, and 70%, then water). To enhance antigen retrieval only for some antibodies, TMA sections were microwave-treated (H2800 Microwave Processor, EBSciences, East Granby, Connecticut, USA) in citrate buffer (Target Retrieval Solution, Dako) at 99°C for 16 min. Endogenous peroxidase activity was blocked by incubating the slides in peroxidase-blocking solution (Dako) for 5 min. The EnVision Detection Kit (Dako) was used as the staining detection system. Sections were counterstained with haematoxilin, dehydrated with ethanol, and permanently coverslipped.
TMA analysis
For each antibody preparation studied, the location of immunoreactivity, percentage of stained cells, and intensity were determined. All the cases were semiquantified for each protein-stained area. An image analysis system with the Olympus BX51 microscope and analysis soft (analySIS®, Soft imaging system, Münster, Alemania) was employed as follows: tumour sections were stained with antibodies according to the method explained above and counterstained with haematoxilin. There are different optical thresholds for both stains. Each core was scanned with a × 400 power objective in two fields per core. Fields were selected searching for the protein-stained areas. The computer program selects and traces a line around antibody-stained areas (higher optical threshold: red spots), with the remaining, non-stained areas (haematoxilin-stained tissue with lower optical threshold) standing out as a blue background. Any field has an area ratio of stained (red) vs non-stained areas (blue). A final area ratio was obtained after averaging two fields. To evaluate immunostaining intensity we used a numeric score ranging from 0 to 3, reflecting the intensity as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, intense staining. Using an Excel spreadsheet, the mean score was obtained by multiplying the intensity score (I) by the percentage of stained cells (PC) and the results were added together (total score: I × PC). This overall score was then averaged with the number of cores that were carried out for each patient. If there was no tumour in a particular core, then no score was given. In addition, for each tumour, the mean score of two core biopsies was calculated.
Furthermore, whole-tissue sections from tumoural blocks from a subset of 10 cases were compared with the corresponding TMA discs, regarding each MMP and TIMP expression. Those cases were selected randomly, and the obtained clinicopathological data were very similar to those from the whole series. Each whole-tissue section was scanned with a × 400 power lens in 10 different fields. Fields were selected searching for the protein-stained areas, as described above.
Data analysis and statistical methods
Immunostaining score values for each protein were expressed as median (range). Comparison of immunostaining values between groups was made with the Mann–Whitney or Kruskall–Wallis tests. Statistical results were corrected applying Bonferroni's correction. For metastasis-free survival analysis, we used Cox's univariate method. Cox's regression model was used to examine interactions of different prognostic factors in a multivariate analysis. Expression profiles were analysed by the unsupervised hierarchical clustering method that organises proteins in a tree structure, on the basis of their similarity. Data were reformatted as follows: −3 designated negative staining, 3 positive staining, missing data was left blank. The score values were reformatted (positive–negative) choosing the median as cutoff value. We used the Cluster 3.0 program (average linkage, Pearson correlation). Results were displayed with Treeview (Eisen et al, 1998). The SPSS 11.5 program was used for all calculations.
Results
More than 2600 determinations in cancer specimens from 131 patients with primary invasive ductal carcinoma of the breast and controls were performed on TMAs. Minimal internal variance of score data between duplicate tissue cores from the same patients was detected in the tissue arrays, showing a high agreement for each protein (r>0.95 and P<0.0001). In the validation study there was total concordance in the global expression, as well as in the intensity of immunostaining, for each MMP and TIMP between TMA cases and the corresponding whole-tissue sections. In addition, there were highly significant correlations in the immunostaining scores between these two paired sets (r>0.90 and P<0.0001, for each protein).
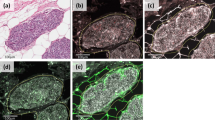
Figure 1 shows examples of TMAs with immunostaining for each protein evaluated. There was a wide variability in the immunostaining score values for each protein (Table 2). Immunostaining for all the proteins studied was localised predominantly in tumour cells, but also in stromal cells in a significant percentage of cases. There were significant associations between the total immunostaining scores for several proteins and clinicopathological parameters of tumoral aggressiveness (Table 2).
Left: examples of TMAs with immunostaining for each protein. 200 × Right: (A) immunohistochemical staining of MMP2 in epithelial cells, (B) TIMP3 in epithelial cells and fibroblastic cells, (C) TIMP3 in inflammatory mononuclear cells, and (D) TIMP2 in epithelial cells, fibroblast and inflammatory mononuclear cells. 400 × .
We initially investigated the possible association between the total immunostaining scores for each MMP and TIMP and the relapse-free survival, taking the median value of the immunostaining score for each protein as the cutoff point. Thus, we found that a high expression of MMP-9 and 11, TIMP-1 and 2 was significantly associated with a shortened relapse-free survival (Table 3 and Figure 2). In addition, our data showed that the expression of MMP-1, 7, 9, 11, 13, 14, TIMP-1, and 2, as a function of the cellular type (tumour cell, fibroblast, and/or inflammatory mononuclear cell) expressing the protein, was significantly associated with a shorter relapse-free survival (Table 3 and Figure 2). Additionally, to identify specific groups of tumours with distinct MMP/TIMP immunohistochemical expression profiles, the data were analysed by unsupervised hierarchical cluster analysis. The algorithm orders proteins on the horizontal axis and samples on the vertical axis based on similarity of their expression profiles. However, this did not produce a dendrogram with a well-defined cluster of tumours (Figure 3).
Kaplan–Meier survival curves as function a of the immunostaining score values of MMP-9 (A), MMP-11 (B), TIMP-1 (C), and TIMP-2 (D); expression by tumoral cells of MMP-9 (E) and TIMP-2 (F); expression by fibroblast cells of MMP-1 (G), MMP-7 (H), MMP-9 (I), MMP-11 (J), MMP-13 (K), TIMP-2 (L), and TIMP-3 (M); expression by mononuclear inflammatory cells of MMP-7 (N), MMP-9 (O), MMP-11 (P), MMP-13 (Q), MMP-14 (R), TIMP-1 (S), and TIMP-2 (T).
Graphical representation of two-dimensional unsupervised hierarchical clustering results on immunohistochemistry expression profiles of 10 proteins in 131 breast cancer samples. Rows: samples; columns, proteins. Protein expression scores are depicted according to a colour scale: red, positive staining; green, neative staining; grey, missing data. Dendogram of samples (to the left of matrix) and proteins (above matrix) represent overall similarities in expression profiles. Status column: 1=with recurrence; 0, without recurrence, at the census point.
Multivariate analysis between classical prognostic factors according to Cox model demonstrated that tumoral stage (II: relative risk (RR) (confidence interval (CI))=1.8(0.9–3.6); III: 3.9(2–8); P<0.001) and PgR status (positive: 0.36(0.2–0.6), P<0.001) were significantly and independently associated with relapse-free survival. All the MMP and TIMP expressions that reached significance for predicting distant metastases in the univariate analysis were significantly and independently associated with relapse-free survival in the multivariate analysis (Table 3).
Discussion
This is, to the best of our knowledge, the first study analysing the expression of MMPs and TIMPs in human breast cancer by applying TMA technology, which allows one to integrate different biological aspects of the tumour in the morphological context of breast carcinoma.
Matrix metalloproteinases -2 (gelatinase A) and MMP-9 (gelatinase B) are related to tumour invasion and metastasis by their special capacity to degrade the type IV collagen found in basement membranes (Jones and Walker, 1997), and to induce angiogenesis (Egeblad and Werb, 2002). Our results are in accordance with those of previous reports showing that a high MMP-9 expression correlates significantly with tumoral aggressiveness and poor prognosis (Chantrain et al, 2004; Li et al, 2004; Pellikainen et al, 2004), as well as with other studies where high MMP-2 expression in carcinoma cells, in contrast, has been related to only a few inverse prognostic factors (Talvensaari-Mattila et al, 1998; Nakopoulou et al, 2003) or shown to have no association with clinicopathological parameters in breast cancer (Jones et al, 1999; Hirvonen et al, 2003; Talvensaari-Mattila et al, 2003). It has also been described that as breast cancer progresses, MMP-2 production increases during the early phases, whereas activation of MMP-9 occurs during the late cancerous stage (Liotta and Kohn, 2001), which could explain their different impact on prognosis in clinically detected invasive breast tumours.
Matrix metalloproteinase-1 (collagenase-1), the most ubiquitously expressed of the interstitial collagenases, is required for local invasion because it possesses the ability to efficiently degrade type I collagen – the principal component of connective tissue (Brinckerhoff et al, 2000). We found that high expression of MMP-1 by fibroblast cells correlated with the occurrence of metastasis, which is in accordance with previous studies showing that this MMP is associated with elevated metastasis capacity (Kang et al, 2003; Prybylowska et al, 2006). Matrix metalloproteinase -7 (matrilysin 1) is a stromelysin, that degrades type IV collagen, fibronectin and laminin. It was shown that MMP-7 is aberrantly expressed in human breast tumours and that elimination of MMP-7 is associated with low invasiveness and slow tumour growth (Jian et al, 2005). Likewise, it has been recently reported that MMP-7 overexpression in breast cancer (MCF-7) cells enhances cellular invasiveness and activation of proMMP-2 and MMP-9 (Wang et al, 2006). However, the potential role of MMP-7 in human breast cancer, and particularly in clinical breast cancer, has not been thoroughly investigated. Our results are in accordance with these experimental studies showing that high intratumoral levels of MMP-7 were significantly associated with several parameters indicatives de tumoral aggressiveness and linked with a high occurrence of distant metastasis.
Similarly to other studies, our data show that MMP-11 (Stromalysin-3) was preferentially expressed by peritumoral stromal cells (Basset et al, 1990; Basset et al, 1997) and that high levels of MMP-11 were associated with tumour progression and poor prognosis (Chenard et al, 1996; Ahmad et al, 1998). Matrix metalloproteinase -13 (collagenase-3) has been found to have an exceptionally wide substrate specificity when compared with other MMPs (Freije et al, 1994; Knauper et al, 1997). Moreover, it is thought to play a central role in the MMP activation cascade, both activating and being activated by several other MMPs (MMP-14, 2, or 3). Nielsen et al (2001) have reported that MMP-13 expression by myofibroblasts was often associated with microinvasive events, and they have proposed that this MMP may play an essential role during the transition of ductal carcinoma in situ lesions to invasive ductal carcinoma of the breast. In the present study, we found high MMP-13 expression in early-stage tumours, but also associated with tumours showing an infiltrating edge and with a higher rate of distant metastases when the MMP was expressed by fibroblastic cells or by inflammatory mononuclear cells. Matrix metalloproteinase -14 (membrane type 1 MMP, or MT1-MMP) is a key metalloprotease involved in the degradation of extracellular matrix, activates pro-MMP-13 (Knauper et al, 1996) and pro-MMP-2 (Strongin et al, 1995) on the cell surface, and plays crucial roles in molecular carcinogenesis, tumour cell growth, invasion, and angiogenesis. In the present study, we found significant associations between the expression of MMP-14 and clinicopathological parameters indicative of tumour aggressiveness. The strong association between MMP-14 expression by stromal cells and poor prognosis described in the present study is also remarkable .
Our results showing a significant association between TIMP-1, TIMP-2 and several parameters indicative of tumoral aggressiveness as well as with a high occurrence of distant metastases are in accordance with similar findings reported by other authors (Ree et al, 1997; Remacle et al, 2000; Schrohl et al, 2004). If TIMPs inhibit MMPs in vivo, it should be expected that high levels of these inhibitors would prevent tumour progression and thus be related with good outcome in patients with cancer. However, TIMPs are multifunctional proteins that in addition to their MMP-inhibitory effect also demonstrate distinct tumour-stimulatory functions (Jiang et al, 2002).
Experimental studies have shown that TIMP-3 may show activity to inhibit angiogenesis and induce apoptosis (Ahonen et al, 1998; Spurbeck et al, 2002). It has also been published that high TIMP-3 mRNA levels are associated with a good prognosis in breast cancer (Kotzsch et al, 2005). Likewise, Span et al (2004) have reported that high levels of TIMP-3 predicted a longer relapse-free survival in patients treated with tamoxifen. All these findings suggest that TIMP-3 is involved in specific pathways of tamoxifen-induced apoptosis. Our results show a significantly higher TIMP-3 expression in ER-positive tumors, in accordance with a prior study (Span et al, 2004). However, we also found that TIMP-3 expression by fibroblastic cells, but not by tumoural cells, correlates positively with the occurrence of distant metastases, reflecting the existence of other mechanisms in the molecular biology of the breast tumours in which this TIMP might be implicated.
In the present study, relevant our finding that the expression of MMP-1, 7, 11, 14, TIMP-1, 2 or 3 by fibroblasts and/or by inflammatory mononuclear cells was significantly associated with a higher incidence of distant metastases was especially , suggesting that the tumoral stroma does not play a merely passive role in cancer progression. In fact, over the past few years evidence has accumulated that both changes in stromal behaviour and the interaction between tumour cells and stromal cells are intimately linked to the processes of tumorgenesis, tumour invasion, and metastasis (Liotta and Kohn, 2001; Klausner, 2002; Wiseman and Werb, 2002). It has been demonstrated that several types of malignant cells (eg, breast and colon) actively recruit fibroblasts into tumours, leading to an increase in the extent of extracellular matrix degradation (Sloane et al, 2005). Likewise, it has been shown that incubation of breast cancer cells with monocytes or macrophages induces a crosstalk that results in an increased expression of factors involved in cancer cell invasiveness and in a modification of the monocytes function against cancer cells (Blot et al, 2003; Pukrop et al, 2006). We could hypothesise that tumours secrete factors able to elicit a wound-repair response from tumour-associated and tumour-infiltrating inflammatory cells, this response inadvertently stimulating tumour progression (Queen et al, 2005); or that it is the host tissue, with a redundant response of biochemical factors to cancerous cells, that induces the tumour growth. Even so, our data indicate a biological variability in the behaviour of these stromal cells with regard to the expression of MMPs and TIMPs, which is of clinical importance.
Our data also show that the expression of some MMPs and TIMPs has a potential value as predictor of distant metastases except for MMP-7 and MMP-14, without lymph node involvement. By contrast, we have surprisingly found that the global expression of TIMP-2 and MMP-13 correlated negatively with lymph node involvement. Altogether, our data support the concept that the biological mechanisms involved in blood vessel and lymphatic dissemination are dependent on different processes within tumoral pathophysiology. Nevertheless, we also have to consider that in the present study, we investigated the intratumoral stroma, and the putative absence of intratumoral lymphatics in invasive breast carcinomas is well known (Vleugel et al, 2004). On the other hand, it is also of note that we did not find well-defined cluster groups with regard to scores of immunostaining values of MMPs and TIMPs, which iis probably due to the biological heterogeneity of breast cancer.
In summary, our results demonstrate the importance of MMPs and TIMPs in the progression of breast cancer, and suggest their value in order to reach a more precise prognostic estimation in invasive ductal carcinoma of the breast.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, Rubens R, Anglard P, Hart I (1998) Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol 152: 721–728
Ahonen M, Baker AH, Kahari VM (1998) Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 58: 2310–2315
Basset P, Bellocq JP, Lefebvre O, Noel A, Chenard MP, Wolf C, Anglard P, Rio MC (1997) Stromelysin-3: a paradigm for stroma-derived factors implicated in carcinoma progression. Crit Rev Oncol Hematol 26: 43–53
Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P (1990) A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 348: 699–704
Blot E, Chen W, Vasse M, Paysant J, Denoyelle C, Pille JY, Vincent L, Vannier JP, Soria J, Soria C (2003) Cooperation between monocytes and breast cancer cells promotes factors involved in cancer aggressiveness. Br J Cancer 88: 1207–1212
Brinckerhoff CE, Rutter JL, Benbow U (2000) Interstitial collagenases as markers of tumor progression. Clin Cancer Res 6: 4823–4830
Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Invest 80: 1943–1949
Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA (2004) Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res 64: 1675–1686
Chenard MP, O'Siorain L, Shering S, Rouyer N, Lutz Y, Wolf C, Basset P, Bellocq JP, Duffy MJ (1996) High levels of stromelysin-3 correlate with poor prognosis in patients with breast carcinoma. Int J Cancer 69: 448–451
Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD (1998) Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 161: 6845–6852
Demers M, Couillard J, Belanger S, St-Pierre Y (2005) New roles for matrix metalloproteinases in metastasis. Crit Rev Immunol 25: 493–523
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174
Eisen MB, Spellman PT, Brown PO, Bolstein D (1998) Cluster analysis and dsiplay of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868
Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM (2001) Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3: 459–468
Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C (1994) Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 269: 16766–16773
Galea MH (1992) Nottingham prognostic grade. Breast Cancer Res Treat 22: 207–249
Hirvonen R, Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T (2003) Matrix metalloproteinase-2 (MMP-2) in T(1–2)N0 breast carcinoma. Breast Cancer Res Treat 77: 85–91
Jian WG, Davies G, Martin TA, Parr CH, Watkins G, Mason MD, Mokbel K, Mansel RE (2005) Targeting matrilysin and its impact on tumor growth in vivo: the potential implications in breast cancer therapy. Clinical Cancer Res 11: 6012–6019
Jiang Y, Goldberg ID, Shi YE (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21: 2245–2252
Jones JL, Glynn P, Walker RA (1999) Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol 189: 161–168
Jones JL, Walker RA (1997) Control of matrix metalloproteinase activity in cancer. J Pathol 183: 377–379
Kang Y, Siegel PM, Shu W, Drobnjak M, Käkönen SM, Cordón-Cardo C, Guise TA, Massagué J (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3: 537–549
Klausner RD (2002) The fabric of cancer cell biology-weaving together the strands. Cancer Cell 1: 3–10
Knauper V, Cowell S, Smith B, Lopez-Otin C, O'Shea M, Morris H, Zardi L, Murphy G (1997) The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem 272: 7608–7616
Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G (1996) Biochemical characterization of human collagenase-3. J Biol Chem 271: 1544–1550
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844–847
Kotzsch M, Farthmann J, Meye A, Fuessel S, Baretton G, Tjan-Heijnen VC, Schmitt M, Luther T, Sweep FC, Magdolen V, Span PN (2005) Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression levels in breast cancer. Eur J Cancer 41: 2760–2768
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS, Di GH, Liu G, Li FM, Ou ZL, Jie C, Shen ZZ, Shao ZM (2004) Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat 88: 75–85
Liotta LA, Kohn EC (2001) The microenvironment of the tumour-host interface. Nature 411: 375–379
Manes S, Llorente M, Lacalle RA, Gomez-Mouton C, Kremer L, Mira E, Martinez AC (1999) The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem 274: 6935–6945
Nakopoulou L, Tsirmpa I, Alexandrou P, Louvrou A, Ampela C, Markaki S, Davaris PS (2003) MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res Treat 77: 145–155
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM (2000) Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18: 1135–1149
Nielsen BS, Rank F, Lopez JM, Balbin M, Vizoso F, Lund LR, Dano K, Lopez-Otin C (2001) Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res 61: 7091–7100
Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118
Overall CM, Lopez-Otin C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2: 657–672
Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB (2002) Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol 117: 723–728
Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM (2004) Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res 10: 7621–7628
Prybylowska K, Kluczna A, Zadrozny M, Krawczyk T, Kulig A, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J, Blasiak J (2006) Polymorphisms of the promoter regions of matrix metalloproteases genes MMP-1 and MMP-9 in breast cancer. Breast Cancer Res Treat 95: 65–72
Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C (2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA 103: 5454–5459
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL (2005) Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res 65: 8896–8904
Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O (1997) High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 3: 1623–1628
Remacle A, McCarthy K, Noel A, Maguire T, McDermott E, O'Higgins N, Foidart JM, Duffy MJ (2000) High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer 89: 118–121
Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, Brunner N, Foekens JA (2004) Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res 10: 2289–2298
Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher ML, Mai J, Cavallo-Medved D, Sameni M, Dosescu J, Moin K (2005) Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Semin Cancer Biol 15: 149–157
Span PN, Lindberg RL, Manders P, Tjan-Heijnen VC, Heuvel JJ, Beex LV, Sweep CG (2004) Tissue inhibitors of metalloproteinase expression in human breast cancer: TIMP-3 is associated with adjuvant endocrine therapy success. J Pathol 202: 395–402
Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM (2002) Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood 100: 3361–3368
Stetler-Stevenson WG (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103: 1237–1241
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338
Talvensaari-Mattila A, Paakko P, Hoyhtya M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T (1998) Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer 83: 1153–1162
Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T (2003) Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer 89: 1270–1275
Turk V, Kos J, Turk B (2004) Cysteine cathepsins (proteases) – on the main stage of cancer? Cancer Cell 5: 409–410
Vleugel MM, Bos R, van der Groep P, Greijer AE, Shvarts A, Stel HV, van der Wall E, van Diest PJ (2004) Lack of lymphangiogenesis during breast carcinogenesis. J Clin Pathol 57: 746–751
Wang F, Reierstad S, Fishman DA (2006) Matrilysin over-expression in MCF-7 cells enhances cellular invasiveness and pro-gelatinase activation. Cancer Lett 236: 292–301
Wiseman BS, Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049
Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H, Brunner N (2005) Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 12: 215–227
Acknowledgements
We thank Dr Miguel Bongera for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant support: This work was supported by grants from: Fondo de Inversión Sanitaria del Instituto Carlos III (FIS-PI040137) (FIS-Spain), Red de Centros de Cáncer RTICCC (C03/10) and Obra Social Cajastur.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Vizoso, F., González, L., Corte, M. et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer 96, 903–911 (2007). https://doi.org/10.1038/sj.bjc.6603666
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603666
Keywords
This article is cited by
-
HSP47 promotes metastasis of breast cancer by interacting with myosin IIA via the unfolded protein response transducer IRE1α
Oncogene (2020)
-
MMP-11 as a biomarker for metastatic breast cancer by immunohistochemical-assisted imaging mass spectrometry
Analytical and Bioanalytical Chemistry (2019)
-
Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells
BMC Complementary and Alternative Medicine (2018)
-
Mature and progenitor endothelial cells perform angiogenesis also under protease inhibition: the amoeboid angiogenesis
Journal of Experimental & Clinical Cancer Research (2018)
-
Multimodal laser ablation/desorption imaging analysis of Zn and MMP-11 in breast tissues
Analytical and Bioanalytical Chemistry (2018)