Abstract
Nasopharyngeal carcinoma (NPC) is an Epstein–Barr virus-associated disease with high prevalence in Southern Chinese. Using multiparametric flow cytometry, we identified significant expansions of circulating naïve and memory CD4+CD25high T cells in 56 NPC patients compared with healthy age- and sex-matched controls. These were regulatory T cells (Treg), as they overexpressed Foxp3 and GITR, and demonstrated enhanced suppressive activities against autologous CD4+CD25− T-cell proliferation in functional studies on five patients. Abundant intraepithelial infiltrations of Treg with very high levels of Foxp3 expression and absence of CCR7 expression were also detected in five primary tumours. Our current study is the first to demonstrate an expansion of functional Treg in the circulation of NPC patients and the presence of infiltrating Treg in the tumour microenvironment. As Treg may play an important role in suppressing antitumour immunity, our findings provide critical insights for clinical management of NPC.
Similar content being viewed by others
Main
Nasopharyngeal carcinoma (NPC) is an Epstein–Barr virus (EBV)-associated malignant disease with high prevalence in Southern Chinese. Despite favourable responses to radio-chemotherapy in most patients with early disease, a significant number of patients present with metastatic or refractory disease or develop relapses (Teo et al, 2006). Thus, alternative therapeutic approaches need to be established. One approach is the development of immunotherapy specifically targeting NPC cells for immune destruction by EBV-specific cytotoxic T lymphocytes. Thus far, limited studies reported variable clinical responses (Chua et al, 2001; Comoli et al, 2005; Straathof et al, 2005).
CD4+CD25+ regulatory T cells (Treg) can suppress the activation and proliferation of CD4+ and CD8+ T cells (Piccirillo and Shevach, 2001; Woo et al, 2002) and play an important role in controlling autoreactive T cells. However, for several types of human cancer, an increase of Treg in the peripheral blood (PB) and the presence of Treg at the tumour site has been reported (Woo et al, 2001, 2002; Liyanage et al, 2002; Wolf et al, 2003; Ormandy et al, 2005; Schaefer et al, 2005), indicating that these cells may also suppress T-cell-mediated antitumour immunity (Piccirillo and Shevach, 2001; Curiel et al, 2004). Understanding the role of Treg in NPC patients has important implications for disease management, including the development of successful T-cell immunotherapy.
Materials and methods
Patient recruitment
With informed consent, a total of 57 poorly differentiated and undifferentiated NPC patients were prospectively recruited at diagnosis at the Prince of Wales Hospital (2005–2006), including four patients with stage I tumour, 18 with stage II, 17 with stage III, and 18 with stage IV tumours. All of the tumours were confirmed to be EBV-positive by EBER in situ hybridisation. Five patients were recruited for tumour biopsies collection. For control group, 56 sex- and age-matched healthy blood donors from the Hong Kong Red Cross Blood transfusion Service were used.
Multicolour flow cytometric analysis
Peripheral blood mononuclear cells (PBMNC) were isolated from freshly drawn blood by centrifugation on Ficoll–Hypaque gradient solution (Pharmacia, Uppsala, Sweden). Tumour biopsies were dissociated into single cell suspensions with collagenase digestion and/or mechanical dissociation. The PBMNC and suspended cells were immunostained with fluorochrome-conjugated anti-human antibodies for 25 min at 4°C. For intracellular antigens, the cells were fixed and permeabilised with Becton Dickinson™ (BD) fix/perm solution (BD Biosciences, Bedford, MA, USA) before immunostaining. The panel of antibody combinations conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-cyanin 5 (PECy5), and allophycocyanin (APC)/AlexaFluor647 (AF647) were (1) CD25-FITC/CD3-PE/CD4-PECy5/CD8-APC, (2) CD25-FITC/CD45RO-PE/CD4-PECy5, (3) CD25-FITC/CCR7-PE/CD4-PECY5/CD152-APC, and (4) CD25-FITC/GITR-PE/CD4-PECy5/Foxp3-AF647. Cell acquisition was performed using a BD FACSCalibur flow cytometer. Data of ⩾200 000 lymphocytes gated by forward and side scatter properties were collected for the examination of various marker expressions in the cells. Flow data were analysed using FlowJo© Analysis Software (Treestar Inc., Ashland, OR, USA) for gating different populations of T-cell subsets and determination of expression levels of various markers in Treg cells. Various T-cell subset data as the cell percentage in total lymphocytes and the marker expression levels of CD4+CD25 Treg cells between NPC patients and sex-and age-matched healthy control were statistically analysed using Mann–Whitney U test for nonparametric independent group comparisons.
T-cell proliferation suppression assay
CD4+ T-cells were purified from PBMNC of five patients and four healthy blood donors with negative-immunomagnetic sorting using untouched CD4+ T-cell kit according to manufacturers' recommended protocol (Miltenyi, Germany). The cells were further positive-immunosorted using CD25 microbeads (Miltenyi) to isolate the CD4+CD25+ T-cell population. The CD4+CD25- T-cells were collected in the negative fraction. The CD4+CD25− T-cells (2 × 105/ml) were plated onto BioCoat T-cell activation plates (BD Biosciences, Bedford, MA, USA). Autologous CD4+CD25+ T cells were added at ratios of 1 : 0, 1 : 1, 1 : 2, 1 : 5, and 0 : 1 to CD4+CD25− T cells. After 4 days, satisfactory cell viability was confirmed by using trypan blue exclusion method, and T-cell proliferation was assessed in triplicate by WST-1 cell proliferation assay (Roche, Germany).
Results and discussions
Reduced circulating CD4+ T cells in NPC patients
In this study, we first characterised the T-cell populations in PB of NPC patients. As shown in Table 1, the percentage of CD4+ (P=0.0321) but not CD8+ T cells were significantly decreased in NPC patients as compared with those in sex- and age-matched healthy controls. As the lymphocyte counts of NPC patients (1.7±0.6 × 109/l) were similar to those in the controls (1.8±0.4 × 109/l), this represents a reduction in CD4+ T-cell numbers in the PB of NPC patients. This was true for CD4+ cells in both memory CD4+CD45RO+ (P=0.0005) and naïve CD4+CR45RO− (P=0.0041) T-cell populations (Table 1).
Expansion of circulating CD4+CD25high regulatory T cells in NPC patients
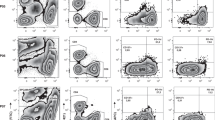
Despite this reduction in the number of circulating CD4+ T cells in NPC patients vs healthy donors, we found an increase in the subset of circulating CD4+ T cells that expressed CD25 (P=0.0001). No such increase was seen for CD8+CD25+ T cells (P=0.4366) (Table 1 and Figure 1A–D). A previous study has demonstrated elevated serum transforming growth factor-β (TGF-β) levels in NPC patients (Xu et al, 1999). Thus, it is of special interest to note that TGF-β can generate and expand CD4+CD25+ Treg from human PB (Yamagiwa et al, 2001). To exclude the population of activated T cells, which might express low levels of CD25 in human PB (Baecher-Allan et al, 2004), cells expressing high levels of CD25 were regated and quantified as Treg. Again, significant increase in CD4+CD25high Treg was observed in the patients (Table 1 and Figure 1E). This increase did not correlate with NPC staging, tumour size, lymph node status, or the presence of metastasis (data not shown).
Characterisation of the Treg populations in PB of NPC patients and normal healthy blood donors. Gating of CD4+CD25+ (R2 and R3 gated regions) and CD4+CD25high (R3 gated region) T cells of representative normal individual control (A) and NPC patient (B) are presented. The Foxp3-expressed T cells (coloured in black) were back-gated among the lymphocytes (coloured in grey) in the FASC plots. Percentages of circulating CD3+CD4+CD25+ T cells (C) and CD3+CD8+CD25+ T cells (D) in lymphocytes were determined in 56 NPC patients (▪) and 56 age- and sex-matched normal controls (▴). The CD3+CD4+CD25high T-cell populations were also gated and the percentages between two groups were compared (E). The horizontal lines represent the mean values of the cell percentages. Expression levels of various Treg markers such as CTLA-4/CD152 (F), GITR (G), and Foxp3 (H) in the cells were also examined. Expression levels in terms of mean fluorescence intensity of these markers in representative cases of normal (yellow line) and NPC patients (green line) are shown. The differences in mean values of two groups were statistically analysed by Mann–Whitney U test and the significance level is set at 0.05. Asterisks indicate that the mean percentages of T-cell subsets in lymphocytes of NPC patients showed statistically significant differences as compared to those in controls. (I) Functional characterisation of circulating Treg in healthy volunteers (N=4) and NPC patients (N=5, four from the group of 56 patients and one was also analysed for TIL but not for PB Treg). CD4+CD25− T cells (2 105/ml) were challenged with CD3 antibody and levels of cellular proliferation determined in the presence or absence of autologous Treg added at the ratios indicated. Mean levels of cellular proliferation are expressed as a percentage of that seen in cultures without the addition of Tregs. The mean percentages of CD4+CD25+ Treg, which can achieve 50% inhibition of autologous CD4+CD25− T-cell proliferation, were compared (right). The standard deviations of the percentages were presented as ‘T’ bars.
Characterisation of circulating Treg in NPC patients
Using multiparametric flow cytometric analyses, we demonstrated that the majority (∼90%) of CD4+CD25high cells in both NPC patients and controls expressed various intracellular and surface biomarkers characteristic of Treg. These biomarkers included the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4; CD152), the glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR), and the forkhead box transcriptional factor (Foxp3) (Table 1b). Expression of these markers by CD4+CD25high cells strongly suggests that they are Treg. We also noted that levels of expression of GITR and Foxp3 were significantly higher in CD4+CD25high cells from patients than controls (Figure 1F–H; Table 1b).
It has been demonstrated that Treg is anergic, and in vitro inhibition of CD4+CD25− T-cell proliferation by purified human CD4+CD25+ Treg is linked to their upregulation of Foxp3 mRNA and protein (Baratelli et al, 2005). Thus, we further examined and compared the suppressive activity of CD4+CD25+ Treg cells in five NPC patients and four healthy volunteers. We confirmed the anergic nature of the Treg in both NPC patients and controls. More importantly, we found that the doses of Treg which could achieve 50% inhibition of autologous CD4+CD25− T-cell proliferation were significantly lower (P=0.0159) for NPC patients (23.89±9.44%) than for controls (60.40±6.87%) (Figure 1I). The increased suppressive activity seen in CD4+CD25+ T cells from NPC patients may reflect the greater number of CD4+CD25high cells and/or the increased expression of Foxp3 and GITR in these cells compared with controls.
It was believed that CD4+CD25high Treg belonged to the memory T-cell compartments (Dieckmann et al, 2001; Taams et al, 2001). However, we found that the expanded Treg not only exhibited CD45RO+ memory phenotype (∼70%), as in previous studies (17–18), but also CD45RO- naïve phenotype (∼30%) (Table 1a). Interestingly, Beyer et al (2006) recently also demonstrated an in vivo peripheral expansion of naïve CD4+CD25high Foxp3+ Treg in multiple myeloma patients.
CD4+ T cells expressing the chemokine receptor CCR7 are able to migrate to secondary lymphoid organs and/or tissue (Bromley et al, 2005). Thus, to determine the migratory phenotype of the expanded circulating Treg in our NPC patients, we studied their expression of CCR7. Our data revealed that 93.7 and 92.2% of circulating Treg, respectively, in controls and NPC patients expressed similar levels of CCR7 (Table 1b), indicating a lymph node-homing capacity for the expanded Treg in our NPC patients.
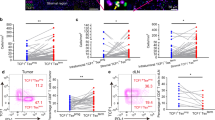
To determine if Treg infiltrated the tumour site, we examined the tumour infiltrating lymphocytes (TIL) in NPC biopsies from five patients. We found that 10.96±1.09% of TIL exhibited a CD4+CD25highFoxp3+ Treg immunophenotype. Interestingly, levels of Foxp3 expression in CD4+CD25high TIL were considerably higher than those seen in circulating CD4+CD25high cells. Furthermore, CD4+CD25high TIL did not express CCR7. (Figure 2; Table 2). The relatively low numbers of TIL that can be obtained from NPC biopsies did not allow functional tests on this population, but it is interesting to speculate that elevated levels of Foxp3 expression correlates with increased suppressive activity. The absence of CCR7 indicates that the migratory properties of these cells has been changed, so that they no longer home to lymph nodes but now migrate to the tumour tissue
Characterising Treg in TIL and PB. The biopsy specimen was mechanically dissociated, fixed, and immunostained with the antibodies to CD4, CD25, and Foxp3 or CCR7. The suspended cells were analysed by flow cytometry gating on lymphocytes. Flow cytometric analysis on PB was as previously described in Figure 1. Two representative cases (NPC1 and NPC2) are shown. The percentage of Tregs was explored in TIL(▪) and PB (□) analysing the CD4+CD25+ gated population (R2 and R3) and the CD4+CD25high population (R3 alone). Expression of Foxp3 and CCR7 in CD4+CD25high Treg from both TIL and PB are presented as histograms. For these histograms, they are the composite of two different FACS plots, one from the data of PB (white coloured peak) and another from TIL (black coloured peak), highlighting the loss of CCR7 expression and increased Foxp3 expression in the CD4+CD25high of TIL as the shifts of those peaks. The horizontal line represents the cutoff of positivity defined using an isotype-matched control antibody.
In conclusion, we have demonstrated an increase of Foxp3+CD4+CD25high Treg in PB and tumour sites in NPC patients. The expanded Treg in the circulation also showed enhanced suppressive activity on CD4+CD25− T-cell proliferations. The increase of this functional Treg population might reduce T-cell-mediated antitumour immunity as represented by the significant decrease in CD4+ T-cell populations in the NPC patients. Thus, the present findings have provided important information and insight into the future design of immunotherapeutic strategies for NPC.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baecher-Allan C, Viglietta V, Hafler DA (2004) Human CD4+CD25+ regulatory T cells. Semin Immunol 16: 89–98
Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175: 1483–1490
Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL (2006) In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 107: 3940–3949
Bromley SK, Thomas SY, Luster AD (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6: 895–901
Chua D, Huang J, Zheng B, Lau SY, Luk W, Kwong DL, Sham JS, Moss D, Yuen KY, Im SW, Ng MH (2001) Adoptive transfer of autologous Epstein–Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer 94: 73–80
Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, Schiavo R, Secondino S, Frasson C, Perotti C, Moroni M, Locatelli F, Siena S (2005) Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein–Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol 23: 8942–8949
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949
Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193: 1303–1310
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169: 2756–2761
Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F (2005) Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 65: 2457–2464
Piccirillo CA, Shevach EM (2001) Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol 167: 1137–1140
Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL (2005) Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 92: 913–920
Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK, Rooney CM, Heslop HE (2005) Treatment of nasopharyngeal carcinoma with Epstein–Barr virus’specific T lymphocytes. Blood 105: 1898–1904
Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN (2001) Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol 31: 1122–1131
Teo PM, Leung SF, Tung SY, Zee B, Sham JS, Lee AW, Lau WH, Kwan WH, Leung TW, Chua D, Sze WM, Au JS, Yu KH, O SK, Kwong D, Yau TK, Law SC, Sze WK, Au G, Chan AT (2006) Dose–response relationship of nasopharyngeal carcinoma above conventional tumoricidal level: a study by the Hong Kong nasopharyngeal carcinoma study group (HKNPCSG). Radiother Oncol 79: 27–33
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9: 606–612
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61: 4766–4772
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH (2002) Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168: 4272–4276
Xu J, Menezes J, Prasad U, Ahmad A (1999) Elevated serum levels of transforming growth factor beta1 in Epstein–Barr virus-associated nasopharyngeal carcinoma patients. Int J Cancer 84: 396–399
Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA (2001) A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol 166: 7282–7289
Acknowledgements
We thank our NPC patients, all the staff at the Molecular Hematology Laboratory, Hematology Section, Department of A & C Pathology at PWH and HKRCBTS for their support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by the Chinese University of Hong Kong, Direct Grant for Research Ref 2005.1.003 Project 2041174, Kadoorie Charitable Foundations (Project ID 6901733) and the Li Ka Shing Institute of Health Science and HKUST 2/03C.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lau, KM., Cheng, S., Lo, K. et al. Increase in circulating Foxp3+CD4+CD25high regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer 96, 617–622 (2007). https://doi.org/10.1038/sj.bjc.6603580
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603580
Keywords
This article is cited by
-
First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors
Journal of Hematology & Oncology (2023)
-
Clinical efficacy and biomarker analysis of dual PD-1/CTLA-4 blockade in recurrent/metastatic EBV-associated nasopharyngeal carcinoma
Nature Communications (2023)
-
Regulatory and effector T cell subsets in tumor-draining lymph nodes of patients with squamous cell carcinoma of head and neck
BMC Immunology (2022)
-
The molecular march of primary and recurrent nasopharyngeal carcinoma
Oncogene (2021)
-
Predictive Value of CD8 Expression and FoxP3 Methylation in Nasopharyngeal Carcinoma Patients Treated with Chemoradiotherapy in a Non-endemic Area
Pathology & Oncology Research (2020)