Abstract
In a 25-year follow-up study of 44 864 men with measured serum cholesterol levels, the testicular cancer hazard ratios for the serum cholesterol categories 5.7–6.9 and ⩾7.0 mmol l−1 vs the reference category (<5.7 mmol l−1) were 1.3 and 4.5, respectively; P-value for trend=0.005. This highly significant association suggests that high-serum cholesterol is a risk factor for testicular cancer.
Similar content being viewed by others
Main
An increased cancer risk has been reported at both high (e.g. Törnberg et al, 1986; Yamada et al, 1998) and low (e.g. Eichholzer et al, 2000) serum cholesterol concentrations. To our knowledge, the relation between testicular cancer risk and serum cholesterol has not been evaluated. Testicular cancer is the commonest cancer site in men aged 15–44 years in Sweden as well as in many other countries. The incidence of testicular cancer has increased in recent decades although the incidence is relatively low, 5.8 per 100 000 in Sweden 2002. Nondescended testes at birth and other abnormal testicular developments are well-established risk factors for testicular cancer (Scottenfeld and Fraumeni, 1996, pp 1213). An ecological correlation between testicular cancer and fat consumption has been demonstrated (Armstrong and Doll, 1975) and case–control studies have also shown an increased risk associated with a high intake of total fat, saturated fat, dietary cholesterol (Sigurdson et al, 1999) and dairy products (Davies et al, 1996; Garner et al, 2003). We have investigated the relation between serum cholesterol and testicular cancer using data from the Värmland cohort.
Materials and methods
Between 1963 and 1965 a mass screening health trial, ‘the Värmland survey’, was conducted in Sweden for a large cohort of 92 710 individuals aged 17–74 years to identify early-stage diseases in an unselected population (Törnberg et al, 1989). Among other measures, blood chemistry analysis including non-fasting serum cholesterol was included in the survey. Earlier findings based on the data from this cohort (Törnberg et al, 1989) accorded with results of other studies, such as the well-known association between serum cholesterol level and coronary heart disease.
The national registration system in Sweden using personal code numbers for all residents makes it possible to link registries to one another. Thus, the cohort data were matched with the Swedish Cancer Registry and the Swedish Cause of Death Registry. Matched data were examined for records of cancer registration and death between 1958 and 1987, giving a 25-year period of follow-up. A more detailed delineation of the cohort has been reported elsewhere (Törnberg et al, 1989).
Subjects with reported cancer (at any site) before they were examined within the survey were excluded from the study population. To avoid the possibility of incorrect conclusions because of inverse causality, that is, that the result is a reflection of preclinical testicular cancer, the cases that occurred within 2 years from the start of the follow-up period were excluded. For statistical analysis, Cox's proportional hazard model was used, with months from serum cholesterol test to testicular cancer event recorded as the follow-up time variable. Observations with no cancer event were censured at the time of death or end of the follow-up period. Hazard ratio (HR) estimates with 95% confidence intervals (CI) and two-tailed statistical tests of significance were computed using the Cox's regression model. In the analysis, serum cholesterol was classified into three categories, namely, serum cholesterol level <5.7, 5.7–6.9 and ⩾7.0 mmol l−1. In the fitted regression model, the cholesterol categories were treated as an indicator variable, with the lowest category as a reference group. The regression model was adjusted by age (5-year groups). A trend test was also included in the analysis.
Results
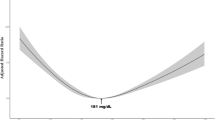
Among the 44 864 men at risk in the cohort, there were 21 cases of testicular cancer during the follow-up period. A positive correlation between serum cholesterol level and testicular cancer incidence was found and the estimated HRs for the middle and highest serum cholesterol categories compared to the lowest was 1.3 (95% CI: 0.3–5.1) and 4.5 (95% CI: 1.3–16.2), respectively (P=0.005) (Table 1).
Discussion
Since high intake of saturated fat or meat is known to elevate the serum cholesterol concentration (Thorogood et al, 1990), the results corroborate the hypotheses advanced concerning a relation between fat intake and testicular cancer (Armstrong and Doll, 1975; Davies et al, 1996; Sigurdson et al, 1999; Garner et al, 2003). Nevertheless, the interpretation of the cholesterol–testicular cancer association may be complicated by other influencing and confounding factors. One example is under-nutrition, the essence of the ‘foetal origins’ hypothesis, which suggests that several adult diseases may be caused by under-nutrition in utero (Godfrey and Barker, 2000). In conformity with this hypothesis, studies have shown that high cholesterol levels (Davies et al, 2004) and testicular cancer (Akre et al, 1996; Moller and Skakkebaek, 1997) are associated with low birth weight. Available data suggest that serum cholesterol concentration in Swedish men decreased during the final decades of the past century (Jansson et al, 2003). Thus, changes in serum cholesterol concentration do not explain the increasing incidence rate of testicular cancer in recent decades.
In conclusion, the highly significant positive association between serum cholesterol and testicular cancer risk found in this population-based cohort study suggests that an elevated concentration of serum cholesterol is a risk factor for testicular cancer. However, since the finding is the first of its sort and because of the wide CIs, more data from other cohorts are needed to confirm the association.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Akre O, Ekbom A, Hsieh CC, Trichopoulos D, Adami HO (1996) Testicular nonseminoma and seminoma in relation to perinatal characteristics. J Natl Cancer Inst 88: 883–889
Armstrong B, Doll R (1975) Enviromental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 15: 617–631
Davies AA, Smith GD, Ben-Shlomo Y, Litchfield P (2004) Low birth weight is associated with higher adult total cholesterol concentration in men: findings from an occupational cohort of 25 843 employees. Circulation 110: 1258–1262
Davies TW, Palmer CR, Ruja E, Lipscombe JM (1996) Adolescent milk, dairy product and fruit consumption and testicular cancer. Br J Cancer 74: 657–660
Eichholzer M, Stahelin HB, Gutzwiller F, Ludin E, Bernasconi F (2000) Association of low plasma cholesterol with mortality for cancer at various sites in men: 17-y follow-up of the prospective Basel study. Am J Clin Nutr 71: 569–574
Garner MJ, Birkett NJ, Johnson KC, Shatenstein B, Ghadirian P, Krewski D (2003) Dietary risk factors for testicular carcinoma. Int J Cancer 106: 934–941
Godfrey KM, Barker DJ (2000) Fetal nutrition and adult disease. Am J Clin Nutr 71: 1344S–1352S
Jansson JH, Boman K, Messner T (2003) Trends in blood pressure, lipids, lipoproteins and glucose metabolism in the Northern Sweden MONICA project 1986–99. Scand J Public Health Suppl 61: 43–50
Moller H, Skakkebaek NE (1997) Testicular cancer and cryptorchidism in relation to prenatal factors: case–control studies in Denmark. Cancer Causes Control 8: 904–912
Scottenfeld D, Fraumeni JF (1996) Cancer Epidemiology and Prevention. Testicular Cancer. Oxford: Oxford University Press
Sigurdson AJ, Chang S, Annegers JF, Duphorne CM, Pillow PC, Amato RJ, Hutchinson LP, Sweeney AM, Strom SS (1999) A case–control study of diet and testicular carcinoma. Nutr Cancer 34: 20–26
Thorogood M, Roe L, McPherson K, Mann J (1990) Dietary intake and plasma lipid levels: lessons from a study of the diet of health conscious groups. Br Med J 300: 1297–1301
Törnberg SA, Holm LE, Carstensen JM, Eklund GA (1986) Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N Engl J Med 315: 1629–1633
Törnberg SA, Holm LE, Carstensen JM, Eklund GA (1989) Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst 81: 1917–1921
Yamada K, Araki S, Tamura M, Sakai I, Takahashi Y, Kashihara H, Kono S (1998) Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol 27: 794–798
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wiréhn, AB., Törnberg, S. & Carstensen, J. Serum cholesterol and testicular cancer incidence in 45 000 men followed for 25 years. Br J Cancer 92, 1785–1786 (2005). https://doi.org/10.1038/sj.bjc.6602539
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602539
Keywords
This article is cited by
-
MicroRNA: a connecting road between apoptosis and cholesterol metabolism
Tumor Biology (2016)
-
Is cholesterol a mediator of cold-induced cancer?
Tumor Biology (2016)
-
Tallness is associated with risk of testicular cancer: evidence for the nutrition hypothesis
British Journal of Cancer (2008)
-
Treatment-related differences in cardiovascular risk factors in long-term survivors of testicular cancer
Journal of Cancer Survivorship: Research and Practice (2007)