Abstract
Most small cell lung cancers (SCLC) coexpress the c-kit protein tyrosine receptor kinase and its ligand stem cell factor, resulting in an autocrine loop. As SCLC growth is also driven by insulin-like growth factor-1 receptor (IGF-1R) signalling, tyrphostins AG 1024 and 1296 (inhibitors of IGF-1R and c-kit activity, respectively) were used to co-target these receptors in H 209 SCLC cells. Combination treatment caused synergy in proliferation inhibition and in apoptosis induction, and also enhanced reduction in phosphorylation of Erk1/Erk2, suggesting that co-targeting IGF-1R and c-kit in SCLC may be more effective than single-agent therapies.
Similar content being viewed by others
Main
The signalling activity of receptor protein tyrosine kinases (PTKs) tightly controls crucial cellular processes such as apoptosis, differentiation and proliferation, and it is therefore not surprising that deregulation of these molecules often leads to neoplastic progression. The involvement of PTKs in the pathology of many cancers has brought attention to these receptors as potential targets for therapeutic intervention (Morin, 2000; Shawver et al, 2002; Dancey and Sausville, 2003). Some neoplastic conditions result from excessive activity of a single PTK (Bcr-Abl in chronic myeloid leukaemia (Melo, 1996), c-kit or PDGFRA in gastrointestinal stromal cell tumours (Hirota et al, 1998; Heinrich et al, 2003)), and are effectively treated using the PTK inhibitor Imatinib (STI 571, Gleevec) (Druker et al, 2001; van Oosterom et al, 2001). However, most cancers involve dysfunction of more than one PTK and crosstalk between their downstream signalling pathways. A therapeutic approach to address this issue involves co-targeting different PTKs (Wu et al, 1995; Ye et al, 1999; Moasser et al, 2001; Moulder et al, 2001; Lu et al, 2001; Chakravarti et al, 2002; Totpal et al, 2002; Camirand et al, 2002). For co-targeting approaches to be useful, careful consideration must be given to the choice of PTKs to be simultaneously blocked (Dancey and Sausville, 2003).
Small cell lung cancer (SCLC) is an aggressive cancer with poor prognosis, which readily metastasises outside the chest, and has a median survival of approximately 9 months (Chute et al, 1999). SCLC has been treated with traditional combination chemotherapy since the 1970s, but less than 3% of patients with extensive-stage SCLC survive more than 3 years (Chute et al, 1999). The molecular pathology of more than 70% of SCLC cases involves autocrine stimulation due to the coexpression of c-kit and its ligand stem cell factor (SCF) (Hibi et al, 1991), and high c-kit expression level is associated with a poor prognosis (Micke et al, 2003). Imatinib (which targets Bcr-Abl, c-kit, and PDGFR (Morin, 2000)), tyrphostin AG 1296 (which inhibits c-kit and PDGFR (Kovalenko et al, 1994)) and indolinones SU 5416 and SU6597 (inhibitors of c-kit and related kinases (Krystal et al, 2001)) reduce SCLC proliferation in vitro (Krystal et al, 1997, 2000, 2001; Wang et al, 2000; Kijima et al, 2002). Phase I and II clinical trials with Imatinib are ongoing for SCLC.
Another growth pathway known to sustain proliferation in SCLC is the IGF-1R signalling cascade (Nakanishi et al, 1988; Macaulay et al, 1990). In many cancers, IGF-1R signalling leads to activation of PI-3 K and MAPK pathways, stimulates proliferation, promotes angiogenesis and metastasis, and inhibits apoptosis (Khandwala et al, 2000; Pollak, 2000; Yu and Rohan, 2000; Wang and Sun, 2002). Preclinical work has demonstrated that IGF-1R could be used as a successful co-target with EGFR (Chakravarti et al, 2002) and HER2/erbB2 (Lu et al, 2001; Camirand et al, 2002), and in the latter study, synergistic inhibition of proliferation was observed in co-targeted breast cancer cells. The availability of several novel inhibitors of IGF-1R signalling (Ludwig et al, 2003; Garcia-Echeverria et al, 2003; Mitsiades et al, 2003) implies that clinical exploitation of any synergism between co-targeting of c-kit and IGF-1R will be practical.
In this study, we use tyrphostins AG 1024 (IGF-1R inhibitor) and AG 1296 (PDGFR and c-kit inhibitor) to inhibit PTK activity in H 209 SCLC cells which present no PDGFR receptors. We demonstrate that combination treatment causes synergy in inhibition of proliferation and in apoptosis induction in these cells, and we speculate that co-targeting the IGF-1R and c-kit signalling pathways is potentially a more effective approach for this system than single-agent therapies.
Materials and methods
Cell culture and proliferation assays
H 209 SCLC cells were obtained from ATCC (Manassas, VA, USA) and cultured at 37°C with 5% CO2 in RPMI 1640 medium with 10% foetal bovine serum (FBS) (InVitrogen, Gaithersburg, MD, USA) except in growth inhibition assays where the FBS supplement was reduced to 1%. Cell proliferation was measured with the Alamar Blue dye reduction method (Biosource International, Camarilo, CA, USA), which is optimal for cells growing in anchorage-independent conditions. Tests were conducted with 104 cells well−1 in 200 μl media in 96-well plates (edge wells were not used, but filled with 200 μl PBS) and three replicates per dose combination were used for each experiment. Stock solutions (10 mM) in DMSO of tyrphostins AG 1024 and AG 1296 (Calbiochem, San Diego, CA, USA) were kept at −20°C and diluted in 1% medium just before use. DMSO concentration in final culture was kept below 0.2% (v v−1). All procedures involving tyrphostins were conducted in low light intensity.
Detection of surface receptors
H 209 cells were collected by centrifugation and washed with FACS buffer 4°C (PBS containing 3% FBS and 0.02% NaN3). Approximately 106 cells were stained with phycoerythrin (PE)-conjugated anti-IGF-1R, anti-PDGFRα, or PDGFRβ antibodies (BD Pharmingen, San Diego, CA), or with fluorescin (FITC)-conjugated anti-c-kit (CD 117) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were incubated with the antibodies for 30 min at 4°C in the dark, washed twice, and resuspended in FACS buffer. Analysis was conducted for 20 000 cells using a FACSCalibur flow cytometer (BD Biosciences, Burlington, MA, USA) with CellQuest software (BD Biosciences Immunocytometry Systems, Franklin Lakes, NJ, USA). Normal mouse IgG1 (Santa Cruz Biotechnologies) was used for isotype determination.
Cell cycle analysis
Approximately 106 cells were collected by centrifugation, washed twice in PBS and fixed in ice-cold 70% ethanol for 1 h, then stained for 30 min in the dark at 37°C in 1 ml propidium iodide (PI) staining buffer (0.1% Triton X-100, 0.1 mM EDTA pH 7.4 in PBS, to which 50 μg ml−1 PI and 0.05 mg RNAse were added just before use). DNA staining was determined by flow cytometry on a FACSCalibur using CellQuest software (see previous section).
Induction of apoptosis
Approximately 106 cells were collected by centrifugation and washed in PBS, then stained with annexin-V-FITC and with PI using the ApoTarget kit (Biosource International, Camarillo, CA, USA). Analysis was conducted by flow cytometry on a FACSCalibur using CellQuest software (see receptor section above).
Western blots
H 209 cells (approx. 3 million) were treated with various doses of tyrphostins or vehicle for a period of 72 h. Cells were lysed in nondenaturing buffer (1% Nonidet NP-40, 20 mM Tris-Cl pH 8.0, 0.5 mM Na orthovanadate pH 9.0 and proteinase inhibitors (Roche, Mannheim, Germany), and freeze-thawed 3 times. Insoluble material was removed by centrifugation, and 50 μg aliquots of the supernatant were separated on 12% polyacrylamide gels. After transfer to TransBlot nitrocellulose membranes (BioRad, Hercules, CA, USA), the proteins were reacted O/N with the following primary antibodies: anti-Akt, anti-phospho-Akt (ser 473), anti-Erk1/Erk2 (p44/42), and anti-phospho Erk1/Erk2 (Thr202/Tyr204) (Cell Signalling, Beverly, MA, USA) at 1 : 1000 dilution, then 1 h with 1 : 2000 horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Pharmacia-Amersham, Piscataway, MJ, USA). Blots were reacted with the ECL chemoluminescence kit (Pharmacia-Amersham, Piscataway, NJ, USA) and exposed to X-OMAT LS film (Kodak, Rochester, NJ, USA). Densitometry analysis was conducted using the Scion Image software (Scion Corp., Frederick, MD, USA).
Results
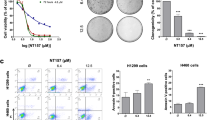
H 209 cells express no PDGF receptors, but present both c-kit (Kijima et al, 2002) and IGF-1R (Nakanishi et al, 1988; Quinn et al, 1996), as analysed by flow cytometry (Figure 1). Treatment with tyrphostins as single agents revealed an IC50 for inhibition of cell proliferation of 3.75±0.8 μ M for AG 1024, and 21.5±3.3 μ M for AG 1296. These two inhibitors were used in a matrix of combinations, and an isobologram plot (Berenbaum, 1981) for IC50 demonstrated synergistic interactions in growth inhibition (Figure 2A). Combination index (CI) values for several combination treatments were calculated from the classic isobologram equation and proved significantly smaller than 1, indicating synergy. Combination treatments showed no synergy of action on the status of any phase of the cell cycle, as observed by flow cytometry after propidium iodide staining of ethanol-fixed cells (results not shown). In contrast, the synergy in growth reduction was observed to parallel a synergy in apoptosis induction (IC75 CI=0.62 at AG 1024 2.5 μ M and AG 1296 5.0 μ M, Figure 2B), pointing to a cytotoxic effect in these cancer cells associated with the co-targeting of IGF-1R and c-kit.
Surface expression of IGF-1R, c-kit, PDGFR α, and PDGFR β on H 209 SCLC cells. H 209 cells collected by centrifugation were stained with phycoerythrin (PE)-conjugated anti-IGF-1R, anti-PDGFR α, anti-PDGFR β antibodies, or fluorescein (FITC)-conjugated anti-c-kit (CD 117) antibody (shaded peaks). Flow cytometry analysis reveals the presence of IGF-1R and c-kit but absence of PDGFR receptors. Normal mouse IgG1 was used for isotype determination (open peaks). Positive controls for anti-PDGFR α and β antibodies were in robust primary cells and TC-71 Ewing's sarcoma cells, respectively (not shown). Counts indicate number of events.
Synergy of IGF-1R and c-kit-targeting treatments. (A) Isobologram plot at IC50 and combination index (CI) of the effects of AG 1094 and AG 1296 on H 209 SCLC cell growth. Tests were conducted in triplicates with 104 cells treated with tyrphostins singly or in combination for a total of 72 h in RPMI with 1% FBS, and proliferation of the anchorage-independent cells was assayed by Alamar blue dye reduction (shown is an experiment representative of five). Proliferation CI values were calculated using the classic isobologram equation (Berenbaum, 1981) and are indicated on a graph. CI values <1 indicate synergy. Single-agent dose–response curves on H 209 cell growth for individual tyrphostins are shown on the right. (B) Apoptosis in H 209 cells treated with tyrphostins AG 1024 (0, 0.25, 2.5 μ M) and AG 1296 (0, 2.5, 5.0 μ M) independently or in combination for a total of 72 h in 3 ml RPMI 1% FBS in six-well plates. Cells were collected by centrifugation, washed in PBS, and stained with annexin V-FITC and PI, and analysed by flow cytometry. Annexin-V-positive cells (M1 peak) represent apoptotic populations. Number above M1 peak is the increase in the percentage of apoptotic cells over the basal control level (Cntrl=34.5%), (shown is one of two experiments producing similar results). (C) Modification of phosphorylation levels of Erk1/Erk2 in H 209 cells treated for 72 h in media containing 1% FBS with 1.0 μ M AG 1024, and/or 5.0 μ M AG 1296. Western blot analysis was conducted on 50-μg protein aliquots with anti Erk1/Erk2 (p44/42) and anti-phospho Erk1/Erk2 (Thr202/Tyr204) primary antibodies and HRP-conjugated anti-rabbit IgG, and revealed with the ECL chemoluminescence reaction. Densitometric analysis of the bands is shown below. C: control cells treated with vehicle (shown is one of two experiments producing similar results).
At concentrations where apoptosis is induced synergistically by combinations of the two inhibitors, co-treatment did not enhance inhibition of Akt phosphorylation over levels observed after treatment with AG 1024 alone (results not shown). However, co-treatment reduced the phosphorylation level of p44/42 (Erk1/Erk2) by 60% with respect to control (Figure 2C).
Discussion
In this study, tyrphostins AG 1024 (IGF-1R inhibitor) and AG 1296 (PDGFR and c-kit inhibitor) were used in combination for treatment of H 209 SCLC cells. Since specificity of tyrphostin action is not strict, (for example, AG 1296 inhibits c-kit, PDGFR α and PDGFR β with similar Kds (Kovalenko et al, 1994)), we demonstrated the absence of PDGF receptors in the target cells, and confirmed the presence of IGF-1R and c-kit. The data presented here indicate that in H 209 SCLC cells, blockade of IGF-1R signalling synergistically enhances the antiproliferative effects of an anti-c-kit strategy, and that while both anti-IGF-1R and anti-c-kit strategies as single agents can induce apoptosis in this system, combination treatment results in synergy of apoptotic induction. Future work will determine whether these observations can be extended to more SCLC cell lines and have relevance to in vivo behaviour of SCLC.
Cellular pathways by which this observed synergy is achieved may involve downstream effectors differentially influenced by the inhibition of IGF-1R and c-kit. Akt/PKB, an important component of the P1-3K-mediated signal route, is involved in several human cancers, and has recently been shown to be important in the progression of lung cancer (Krystal et al, 2002; Chun et al, 2003). Combination treatments did not reduce phosphorylation of Akt below levels obtained by AG 1024 inhibition, however, reduction in the phosphorylation of Erk1/Erk2 was enhanced by co-targeting, suggesting that combination of the IGF-1R and c-kit inhibitors (at lower concentrations than needed for single-agent activity) acts through this kinase to promote the greater-than-additive apoptotic effects observed.
It is tempting to speculate that the antiapoptotic effects of IGF-1 hinder cancer therapies that target other PTKs, and that the antineoplastic effects observed when blocking a PTK may be significantly underestimated if examined only under conditions where IGF-IR is fully functional. Previous work indicates that the in vivo targeting of IGF-1R not only has antineoplastic activity (Arteaga et al, 1989; Andrews et al, 2001) but is well-tolerated (Hofmann et al, 2003). These observations, taken together with our results, suggest that combining anti-IGF-1R and anti-kit targeting may be therapeutically useful in SCLC treatment. Targeted single-agent PTK therapies are effective in cancers where activation by mutation of one particular receptor is responsible for neoplastic progression (Druker et al, 2001; van Oosterom et al, 2001; Heinrich et al, 2002); however, in the more common situations where overexpression or deregulation of multiple PTKs are involved, combination approaches may prove to be of value and the IGF-1 receptor appears as a promising co-target.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andrews DW, Resnicoff M, Flanders AE, Kenyon L, Curtis M, Merli G, Baserga R, Iliakis G, Aiken RD (2001) Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol 19: 2189–2200
Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC, Allred DC, Osborne CK (1989) Blockade of the type l somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest 84: 1418–1423
Berenbaum MC (1981) Criteria for analyzing interactions between biologically active agents. Cancer Res 35: 269–335
Camirand A, Lu Y, Pollak M (2002) Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit 8: BR521–BR526
Chakravarti A, Loeffler JS, Dyson NJ (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res 62: 200–207
Chun K-H, Kosmeder II JW, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee H-Y (2003) Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Nat Cancer Inst 95: 291–302
Chute JP, Chen T, Feigal E, Simon R, Johnson BE (1999) Twenty years of phase III trials for patients with extensive-stage small cell lung cancer: perceptible progress. J Clin Oncol 17: 1794–1801
Dancey J, Sausville EA (2003) Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2: 296–313
Druker BL, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantardjian H, Capdeville R, Ohno-Jones S, Sawyers CL (2001) Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344: 1031–1037
Garcia-Echeverria C, Brueggen J, Capraro H-G, Evans DB, Ferrari S, Fabbro D, Furet P, Geiger T, Liebetanz J, Marti A, Martiny-Baron G, Mestan J, Pearson MA, Ruetz S, Stolz B, Hofmann F (2003) Characterization of potent and selective kinase inhibitors of IGF-1R. Proc AACR 44: 1008
Heinrich MC, Blanke CD, Druker BJ, Corless CL (2002) Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol 20: 1692–1703
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CDM, Fletcher JA (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299: 708–710
Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H, Takahashi T (1991) Co-expression of the stem cell factor and the c-kit genes in small cell lung cancer. Oncogene 6: 2291–2296
Hirota S, Isozaki K, Moriyama Y, Hashimota K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal tumors. Science 279: 577–580
Hofmann F, Brueggen J, Capraro H-G, Cozens R, Evans DB, Fabbro D, Ferrari S, Furet P, Garcia-Echeverria C, Geiger T, Porta DG, Liebetanz J, Maira SM, Marti A, Martiny-Baron G, Mestan J, Meyer T, Ruetz S, Stoltz B, Zimmermann J, Peterson MA (2003) In vitro and in vivo profiling of selective and potent IGF-IR kinase inhibitors. Proc AACR 44: 3798
Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE (2000) The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 21: 215–244
Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R (2002) Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-kit in small cell lung cancer cells. Cancer Res 62: 6304–6311
Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin CH, Waltenberger J, Bohmer FD, Levitzki A (1994) Selective platelet-derived growth factor receptor kinase blockers reverse cis-transformation. Cancer Res 54: 6106–6114
Krystal GW, Carlson P, Litz J (1997) Induction of apoptosis and inhibition of small cell lung cancer growth by the quinoxaline tyrphostins. Cancer Res 57: 2203–2208
Krystal GW, Honsawek S, Kiewlich D, Liang C, Vasile S, Sun L, McMahon G, Lipson KE (2001) Indolinone tyrosine kinase inhibitors block kit activation and growth of small cell lung cancer cells. Cancer Res 61: 3660–3668
Krystal GW, Honwasek S, Litz J, Buchdunger E (2000) The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res 6: 3319–3326
Krystal GW, Sulanke G, Litz J (2002) Inhibition of phosphatidyl-inositol 3-kinase Akt signaling blocks growth, promotes apoptosis and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther 1: 913–922
Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93: 1852–1857
Ludwig D, Burtrum D, Lu D, Anderson DM, Prewett M, Bassi R, Koo H, Jimenez X, Pereira DS, Apblett R, Kussie P, Bohlen P, Hicklin DJ, Zhu Z, Witte L (2003) A fully human monoclonal antibody to the human IGF-1 receptor that blocks ligand-dependant signaling and inhibits the growth of multiple human tumors in nude mice. Proc AACR 44: 761
Macaulay VM, Everard MJ, Teale JD, Trott PA, Van Wyk JJ, Smith IE, Millar JL (1990) Autocrine function for insulin-like growth factor-1 in human small cell lung cancer cell lines and fresh tumor cells. Cancer Res 50: 2511–2517
Melo JV (1996) The diversity of bcr-abl fusion proteins and their relationship to leukemia phenotype. Blood 88: 2375–2384
Micke P, Basrai M, Faldum A, Bittinger F, Ronnstrand L, Blaukat A, Beeh KM, Oesch F, Fischer B, Buhl R, Hengstler JG (2003) Characterization of c-kit expression in small cell lung cancer: prognostic and therapeutic implications. Clin Cancer Res 9: 188–194
Mitsiades CS, Mitsiades N, Kung AL, Garcia-Echeverria C, Pearson MA, Hofman F, Anderson KC (2003) The IGF/IGF-1R system is a major therapeutic target for multiple myeloma, other hematologic malignancies and solid tumors. Proc AACR 44: 4005
Moasser MM, Basso A, Averbuch SD, Rosen N (2001) The tyrosine kinase inhibitor ZD1839 (‘Iressa’) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res 61: 7184–7188
Morin MJ (2000) From oncogene to drug: development of small molecule tyrosine kinase inhibitors as anti-tumor and anti-angiogenic agents. Oncogene 19: 6574–6583
Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL (2001) Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res 61: 8887–8895
Nakanishi Y, Mulshine J, Kasprzyk P, Natale R, Maneckjee R, Avis I, Treston A, Gazdar A, Kramer B, Minna J, Cuttitta F (1988) Insulin-like growth factor-l can mediate autocrine proliferation of human small cell lung cancer lines in vitro. J Clin Invest 82: 354–359
Pollak M (2000) Insulin-like growth factor physiology and cancer risk. Eur J Cancer 36: 1224–1228
Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, Battey J, Mulshine JL, Cuttitta F (1996) Insulin-like growth factor expression in human cancer cell lines. J Biol Chem 271: 11477–11483
Shawver LK, Slamon D, Ullrich A (2002) Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1: 117–123
Totpal K, Lewis Phillips GD, Balter I, Sliwkowski MX (2002) Augmentation of rhuMAb2C4 induced growth inhibition by Tarcevar of the EGFR tyrosine kinase on human breast cancer cell line. Proc AACR 43: 3889
van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, Silberman S, Nielsen OS (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours : a phase I study. Lancet 358: 1421–1423
Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, Stiles CD, Griffin JD, Johnson BE, Salgia R (2000) Growth inhibition and modulation of kinase pathways of small cell lung cancer lines by the novel tyrosine kinase inhibitor STI 571. Oncogene 19: 3521–3528
Wang Y, Sun Y (2002) Insulin-like growth factor receptor-1 as an anti-cancer target: blocking transformation and inducing apoptosis. Curr Cancer Drug Targets 2: 191–207
Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J (1995) Apoptosis induced by an anti-EGFR monoclonal antibody in human colorectal carcinoma cell line and its delay by insulin. J Clin Invest 95: 1897–1905
Ye D, Mendelsohn J, Fan Z (1999) Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human–mouse chimeric anti-EGF receptor mAb C225. Oncogene 18: 731–738
Yu H, Rohan T (2000) Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst 92: 1472–1489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Camirand, A., Pollak, M. Co-targeting IGF-1R and c-kit: synergistic inhibition of proliferation and induction of apoptosis in H 209 small cell lung cancer cells. Br J Cancer 90, 1825–1829 (2004). https://doi.org/10.1038/sj.bjc.6601682
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601682
Keywords
This article is cited by
-
siRNA mediated the type 1 insulin-like growth factor receptor and epidermal growth factor receptor silencing induces chemosensitization of liver cancer cells
Journal of Cancer Research and Clinical Oncology (2008)
-
Lack of CD117 and rare bcl-2 expression in stomach cancer by immunohistochemistry. An immunohistochemical study with review of the literature
Diagnostic Pathology (2006)
-
Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells
Breast Cancer Research (2005)
-
Insulin-like growth factors and neoplasia
Nature Reviews Cancer (2004)