Abstract
Ovarian tumours of low malignant potential (LMP) are intermediate between adenomas and ovarian carcinomas. These tumours are often associated with a significantly better prognosis than ovarian carcinomas. However, a subset of these tumours can progress and become lethal. In order to seek sensitive diagnostic tools for monitoring patients after surgical operation, we performed a genome-wide scan for loss of heterozygosity (LOH) in 41 mucinous LMPs using 91 polymorphic microsatellite markers at an average interval of 50 cM across all of the human chromosomes and 25 LOH markers reportedly associated with ovarian carcinoma. In addition, we assessed whether clinicopathological parameters, microvessel density, Ki-67 labeling index, apoptotic index or p53 overexpression would be useful for predicting the postoperative outcome of LMP patients. Of the 116 markers examined, 19q12 and Xq11–12 showed significant correlation between postoperative progression-free survival time and LOH status (P<0.05). Patients with a high Ki-67 labeling index had a significantly poorer progression-free survival time than those with lower levels (P=0.042). Other clinicopathological factors and immunohistochemical analysis had no correlation with progression-free survival time in this series of patients. When the combination of LOH at 19q12 and/or Xq11–12 was assessed using Cox's regression analysis, patients with tumours that showed LOH at these positions were at greatest risk of progression (P=0.0073). These findings suggest that the identification of LOH at 19q12 and/or Xq11–12 in former mucinous LMP sites should alert the clinician to the presence of a potentially aggressive lesion in the coelomic epithelium, even if a distinction between second primary tumours or recurrence could not be determined.
Similar content being viewed by others
Main
Ovarian carcinoma is the most lethal gynaecological malignancy at present (Wingo et al, 1995). In 1929, Taylor first recognised the existence of a group of ovarian carcinomas that were associated with an improved prognosis; he termed these lesions ‘semimalignant’ tumours (Taylor, 1929). In 1961, the International Federation of Gynaecology and Obstetrics (FIGO) proposed a classification of ovarian tumours that included low malignant potential (LMP) lesions; this designation became effective in 1971 (FIGO, 1987) and was incorporated into the World Health Organisation classification in 1973 (Serov et al, 1973). Characterised by complex branching papillae, epithelial stratification, nuclear atypia, mitotic activity, and absence of stromal invasion, these tumours are considered to be of LMP. Serous and mucinous tumours comprise the vast majority of cases, with endometrioid, clear cell, and Brenner-type tumours also described (Russel et al, 1994). Low malignant potentials (30–50%) are mucinous type (Lee and Scully, 2000) and the incidence of mucinous LMPs is high in Japan compared to Western countries (Nakashima et al, 1990; Tamakoshi et al, 1997). Mucinous LMPs present at earlier stages than invasive ovarian carcinomas and have a more indolent long-term course and excellent prognosis after surgical management alone (Tazelaar et al, 1985; Casey et al, 1993; Trimble and Trimble, 1994; Siriaunkgul et al, 1995; Gershenson, 1999; Lee and Scully, 2000). Mucinous LMP patients (60–85%) who present with stage I disease can be managed conservatively with unilateral salpingo-oophorectomy and complete staging. The postoperation 10-year survival rate is 93–99% for stage I disease (Casey et al, 1993; Trimble and Trimble, 1994; Gershenson, 1999; Lee and Scully, 2000). For the 15–30% of patients who present with stage II–III disease, TAH-BSO and surgical staging are the treatments of choice. Approximately 10–30% of these patients will relapse and 10% will die of the tumour. The criteria for adjuvant chemotherapy for LMP patients are still undetermined. An estimated 10% of patients will develop ovarian carcinoma (Lee and Scully, 2000). Whether these invasive secondary tumours represent progression of LMP to invasive disease or new secondary tumours arising in at-risk populations is still controversial (Siriaunkgul et al, 1995; Lee and Scully, 2000). However, a subset of these tumours can progress and become lethal. This poor outcome is largely due to a lack of sensitive diagnostic tools for monitoring patients after surgical operation, with a reliance upon clinical and pathological parameters that are often difficult to assess for therapeutic decision making. In the search for more reliable prognostic indicators, a few investigators have focused on biological markers that might be predictive of LMP development (Kaern et al, 1990; Padberg et al, 1991). For example, Kaern et al (1990) reported that DNA ploidy pattern was the most important prognostic marker in their study. However, until now it has not been known which molecular markers influence the fate of these patients, because little is known about genetic alterations in LMPs. Among the various types of genetic alterations involved in ovarian carcinoma development and progression, loss of heterozygosity (LOH) of particular chromosomal regions is thought to indicate the deletion of a normally resident tumour suppressor gene (Callahan and Campbell, 1989; Knudson, 1993). Thus, it is possible that the loss of specific alleles may act as diagnostic markers of prognosis. Recently, we reported that allelic loss could be a predictor of poor outcome for ovarian carcinoma patients (Nakayama et al, 2003b). In order to seek potential predictive markers in the current study, we performed a genome-wide scan for LOH in 41 mucinous LMPs using 91 polymorphic microsatellite markers at an average interval of 50 cM throughout all of the human chromosomes (screening markers) and 25 LOH markers reportedly associated with ovarian carcinoma (hotspot markers). In addition, we assessed whether various clinicopathological parameters, microvessel density, Ki-67 labeling index, apoptotic index (AI), or p53 overexpression would be useful for predicting the postoperative outcome of mucinous LMP patients.
Material and methods
Patients and tumour samples
In all, 41 mucinous LMPs and their adjacent non-neoplastic tissues were obtained from archival pathological specimens at the Institute of Japan Surgical Pathology and Seirei Hamatsu General Hospital in Japan. Written informed consent for the analysis in this study was obtained for each case individually. Diagnostic verification and tumour subtyping and grading were performed independently by three certified pathologists (TN, NT, and MF). Low malignant potentials were diagnosed on the basis of conventional histopathologic criteria (Serov et al, 1973), and grading criteria recommended by the International Federation of Gynecology and Obstetrics were used.
The ovarian origin of advanced-stage tumours was confirmed by endoscopic examination, low levels of carcinoembryonic antigen, and the clinical course, as well as by consultation with gastrointestinal specialists of internal medicine and surgery, and urologists. The present tumour tissues consisted of 41 mucinous LMPs. There were 30 stage Ia patients, one stage Ib patients, eight stage Ic patients, two stage II patients, and one stage III patient in this study. Clinicopathological characteristics of these cases are summarised in Table 1.
Unilateral salpingo-oophorectomy and TAH-BSO were performed on patients who presented with stage I disease and with stage II–III disease, respectively. None of the patients received adjuvant chemotherapy. Follow-ups for all patients included in the survival analysis were updated in January 25, 2003 (median follow-up time was 103 months; range, 60–144 months). At that time, six patients had progressed to well-differentiated ovarian carcinoma, one patient had progressed to Pseudomyxoma peritonei, and 34 had not progressed.
DNA extraction
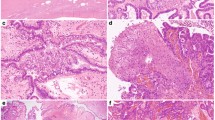
Paraffin-embedded tissues were sectioned at a thickness of 5 μm and stained with haematoxylin and eosin. The cancerous and non-neoplastic portions were collected separately with a 29-gauge needle using an MK1 micromanipulator (Singer Instruments, Roadwater, UK) under a dissecting microscope (Figure 1). The dissected tissue was collected in an Eppendorf tube and incubated overnight at 58°C in a digestion mixture (0.01 M NaCl; 0.5 M Tris-HCl, pH 8.0; 20 mM EDTA; 0.05% Tween-20R; 0.1 mg ml−1 proteinase K). The samples were then heated to 95°C for 10 min to inactivate the proteinase K activity. After the digestion, DNA was extracted with phenol/chloroform treatment and ethanol precipitation. A representative example of LOH is shown in Figure 2.
(A) An LMP tumour before microdissection (× 20, H&E). Arrows indicate stromal cells and connective tissue. *, mucinous substrate without cellular component. Scale bar, 25 μm. (B) The LMP portion after the removal of normal components. The LMP was collected for DNA extraction (× 20, H&E). Arrows indicate removed stromal cells and connective tissue. Scale bar, 25 μm.
Microsatellite markers
We analysed LOH in LMPs with dye-labelled microsatellite markers, including 25 markers for which LOH is reportedly frequent in ovarian carcinoma (hotspot markers). Primer information for the hotspot markers has been described previously (Nakayama et al, 2001). Further LOH analysis in LMPs was carried out with 91 markers located at an average distance of 50 cM apart and distributed throughout all of the human genome (screening markers) (CHLC/Weber Human Screening Set 6A, Research Genetics, Huntsville, AL, USA; primer information can be obtained from K Nakayama).
PCR amplification and analysis of LOH
PCR reactions were performed in a total volume of 10 μl containing 25–50 ng of DNA, dNTPs at a final concentration of 20 μ M, 0.4 μ M of each primer, and 0.25 U of Ex-Taq DNA polymerase (Takara Shuzo, Shiga, Japan) or Platinum Taq DNA polymerase (GIBCO BRL, Rockville, MD, USA). After the mixture was heated for 10 min at 94°C, PCR was performed for 45 cycles at 94°C, at the appropriate annealing temperature and at 72°C for 1 min each, followed by 72°C for 10 min. After denaturation of the PCR products at 94°C for 2 min, samples were subjected to electrophoresis using Performance Optimised Polymer 4 in a 310 Genetic Analyzer (Applied Biosystems, Foster, CA, USA). Loss of heterozygosity analysis was performed by Gene Scan version 2.1. Loss of heterozygosity was quantitatively assessed by calculating the LOH index, which was defined as the allele ratio in the normal tissue divided by the allele ratio in the tumour tissue. The allele ratio was calculated as the peak height of the smaller allele divided by the peak height of the larger allele. If the LOH index was less than 0.5 or more than 2.0, we defined the case as LOH. The LOH frequency of each locus was represented by the ratio of the number of cases with LOH to the total number of informative cases.
Immunohistochemical analysis of p53, Ki-67, apoptosis and microvessel density
Immunohistochemical analysis was conducted by avidin–biotin complex method using 2.5-μm-thick sections from the paraffin-embedded specimens. For the Ki-67 detection, anti-human MIB1 monoclonal antibody (MoAb) (Immunotech S.A., Marseille, France) was used. After blocking the endogenous peroxidase activity, the sections were heated for 20 min in a microwave oven at 500 W to invigorate the antigens of the specimens, as reported by Shi et al (1991) with slight modification. DO-7 and CD34 MoAb's were used to immunostain p53 and microvessels in ovarian carcinomas using the standard immunoperoxidase procedure (Vectastain Elite ABC kit, Vector, Burlingame, CA, USA). Immunostaining and evaluation of p53 and microvessel density were performed as described previously (Nakayama et al, 2002, 2003a). Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling (TUNEL) methodology, using the Apop Tag in situ detection kit (Oncor, Gaithersburg, MD, USA). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labelling methods were performed as described previously (Hata et al, 2002). To obtain the AI (the percentage of immunostained apoptotic tumour cells) and Ki-67 labelling index (Ki-67 LI) (the percentage of immunostained tumour cells), 1000 tumour cells were examined by two observers (KN and YT).
Statistical analysis
Progression-free survival time was measured in months from the month of surgery to the reported recurrence. Survival curves were determined using the Kaplan–Meier method, and differences in survival between subgroups were compared with the log-rank test. P-values less than 0.05 were considered significant. All reported P-values are two-sided.
Results
LOH study
The frequency of LOH for each marker in informative tumours ranged from 0 to 27.3%, with an average of 8.6±4.3% (mean±s.d.). When 13% (mean+1 s.d.) or more of the tumours from informative cases showed LOH, we classified the LOH frequency of the locus as significantly high. Among 116 loci, we observed 22 loci with a significantly high LOH frequency (Table 2).
The most frequent LOH in mucinous LMPs was found at locus D6S503 (6q27; 27.3%).
Clinicopathological factors and immunohistochemical study
The following variables were examined for association with disease progression by Kaplan–Meier methods: age (44> vs 44⩽); stage (Ia vs Ib⩽); CA125 (35> vs 35⩽); and tumour size (15 cm> vs 15⩽) (Table 3). The mean AI of the 41 mucinous LMPs was 1.6% (range, 0–5.6%; median, 1.3%). The proliferative activity of tumour cells is described by the Ki-67 LI in each case. The mean Ki-67 LI of the 41 mucinous LMPs was 28.0% (range, 0.6–77.4%; median, 26.0%). The mean MVD of the 41 mucinous LMPs was 8.3% (range, 3.0–41.0%; median, 6.5%). Overexpression of p53 by immunohistochemistry was observed in 4.2% (two of 41) of analysed tumours. Concerning AI, Ki-67 LI, and MVD, we used the median value (1.3, 26.0, and 6.5) as the optimal cutoff point to divide the patients into two groups (Table 3). As the cases with detectable p53 overexpression were rare, we used positive or negative expression as the optimal cutoff point to divide the patients into two groups.
Progression risk
Of the 116 markers examined, 19q12(D19S433) and Xq11–12 (AR) showed significant correlation between postoperative progression-free survival time and LOH status (Table 3). Kaplan–Meier analysis of progression-free survival time revealed an increased risk of postoperative progression in patients with tumours exhibiting LOH at 19q12 and Xq11–12 compared to patients with tumours that retained both alleles at these loci. As shown in Table 1, we analysed the postoperative progression rate with regard to LOH status at each of the loci, and carried out a log-rank test to evaluate the statistical significance by univariate analysis. None of the other 114 markers tested were correlated with prognosis. Interestingly, these two loci were classified into the significantly high LOH group (16.0%, four of 25, 17.6%, three of 17, respectively). Of the 41 patients with tumours informative at 19q12, 75% of those with LOH progressed within 5 years of surgery, compared to a 5% progression rate among patients with tumours that retained both alleles (P=0.0023, Figure 3A). A correlation between LOH and progression was also observed at Xq11–12, with a 5-year progression rate of 67% in patients with LOH and 5% in patients without LOH (P=0.0122, Figure 3B). Likewise, a combination of 19q12 and/or Xq11–12 revealed a 5-year progression rate of 80% in patients with LOH and 4% in patients without LOH (P<0.0001, Figure 3C). All comparisons were significant (Table 3). In contrast, patients with a high Ki-67 LI had a significantly poorer progression-free survival time than those with lower levels (P=0.042, Table 3). Other clinicopathological factors and immunohistochemical analysis had no correlation with progression-free survival time in this series of patients (Table 3). There was no significant difference in progression-free survival time between intestinal and endocervical type (data not shown). To determine whether these factors were prognostic marker(s) independent of FIGO stage, an established prognostic marker, we conducted a progression-free survival time analysis using the Cox's proportional-hazards model (Table 4). When the combination of LOH at 19q12 and/or Xq11–12 was assessed, patients with tumours who showed LOH at those positions were at greatest risk of progression (P=0.0073, Table 4).
Kaplan–Meier curves for progression-free survival time of patients with tumours that retained both alleles (retention) or that had lost one allele (LOH) at a marker locus. LOH at 19q12 (A), at Xq11–12 (B), and at 19q12 and/or Xq11–12 (C) was significantly associated with progression-free survival time.
Discussion
Mucious LMPs are often associated with a significantly better prognosis than mucinous ovarian carcinomas (Siriaunkgul et al, 1995; Pecorelli and Fallo, 1998; Lee and Scully, 2000). However, gynaecologic oncologists have long sought prognostic markers that could help to assess accurately the postoperative progression risk in mucinous LMP patients, because a subset of these tumours can progress and become lethal. For advanced stage mucinous LMPs, earlier detection of a recurrence may not always result in improved prognosis, due to the lack of efficacious chemotherapy (Tamakoshi et al, 1997). However, in patients with early-stage tumours who have been treated primarily with conservative surgery, earlier detection of recurrence may result in better prognosis. Although until recently routine postoperative decisions for mucinous LMP patients rely on the consideration of conventional prognostic factors such as clinical stage, recently, Winter et al (2002) reported that survival and recurrence rates were not significantly different between staged and unstaged patients who had surgery for LMP patients. Only about half of all patients found to have LMPs undergo complete surgical staging (Link et al, 1996). Removal of the appendix is often included in the staging procedure for ovarian carcinoma, including LMP lesions. The occurrence of synchronous mucinous tumours of the ovary and appendix has been reported (Young et al, 1991; Seidman et al, 1993; Prayson et al, 1994). Recent studies of cytokeratin immunostaining patterns, clonality studies with LOH, and molecular studies of K-ras mutations have supported the conclusion that Pseudomyxoma peritonei usually takes origin from the appendix and not the ovary (Guerrieri et al, 1995, 1997; Cuatrecasas et al, 1996; Szych et al, 1999). In the current study, only one case had a recurrence as Pseudomyxoma peritonei, but we diagnosed it as of ovarian origin because the tumour was restricted to the left ovary and the appendix that was dissected during the surgical operation was intact.
In the present study, we assessed immunohistochemical analysis and various clinicopathological factors that could predict the postoperative progression risk for mucinous LMP patients. However, age, histology, CA125 levels, tumour size, MVD, apoptotic LI, and p53 overexpression did not correlate with postoperative progression rate. Previous studies have suggested that DNA ploidy is prognostically important in mucinous LMP patients (Kaern et al, 1990; Padberg et al, 1991). Kaern et al (1990) and Padberg et al (1991) have correlated DNA aneuploidy with increased tumour progression and shortened patient survival time. In the present study, high Ki-67 LI was significantly associated with tumour progression. Proliferative activity assessed by Ki-67 LI was significantly correlated with DNA aneuploidy in ovarian neoplasm (Huettner et al, 1992). Therefore, Ki-67 LI may indirectly reflect DNA aneuploidy in this series of mucinous LMPs.
Recently, specific LOH patterns have been implicated as predictors of poor survival in various solid tumours (Choi et al, 2000; Hirano et al, 2001; Choi et al, 2002; Rosin et al, 2002; Nakayama et al, 2003b). In the current study, of the 116 markers examined only 19q12 and Xq11–12 showed significant correlation between postoperative progression-free survival time and LOH status (Table 3).
We found that patients whose tumours had shown LOH at 19q12 had a significantly shorter progression-free survival time than those with retention of both alleles at 19q12. Although deletions of chromosome 19q12 have been reported in head and neck carcinoma, gliolbastoma, and astrocytoma, the association of LOH at that region with poor survival has not been reported previously (Nunn et al, 1999; Hu et al, 2002; Beder et al, 2003). We speculate that a candidate tumour suppressor gene in the 19q12 region is associated not only with tumour progression but also with an aggressive clinical phenotype.
At the genetic level, mucinous neoplasms show a characteristic profile that is different from those of serous neoplasms. K-ras mutation is highly characteristic of ovarian mucinous lesions, and may indeed be the defining mutation that confers upon the lesion a mucinous phenotype (Mandai et al, 1998). Schwartz et al (2002) showed that mucinous ovarian carcinomas can be readily distinguished from serous ovarian carcinomas based on their gene expression profiles using oligonucleotide microarrays, regardless of tumour stage and grade (). Furthermore, the LOH profile of mucinous LMPs is similar to that of mucinous ovarian carcinomas, and in tumours showing LMP and invasive carcinoma components, common LOH events have been demonstrated between the LMP and carcinoma areas (Nakayama et al, 2001). These suggest that mucinous LMPs in some cases progress to invasive carcinomas. It has been suggested that deletions that include the AR locus at Xq11.2–12 are involved in the development of both LMP and ovarian carcinoma, including mucinous subtype (Edelson et al, 1998). Thus, mucinous LMP tumours with LOH at that region are precursors of mucinous ovarian carcinoma and result in a poor clinical outcome. Since these two loci discussed above were classified into the significantly high LOH group (16.0%, four of 25, 17.6%, three of 17, respectively, Table 2) in the present allelotype analysis, inactivation of a candidate tumour suppressor gene around these regions could be crucial for the tumourigenesis of mucinous LMP.
This study had several limitations. One limitation is that the number of patients studied was relatively small because of the logistics of long-term patient follow-up. Another limitation is that Pseudomyxoma peritonei related to mucinous LMP that is more likely to be associated with advanced stage, recurrence, and poor prognosis (Nakashima et al, 1990; Casey et al, 1993) was included in only one case in this study. A third limitation is that the multifocality of metastatic disease was included only one case in this study. The fourth limitation is that LMPs with microinvasion was not included in this series of tumours. Larger studies with more cases, including Pseudomyxoma peritonei and LMPs with microinvasion, are needed to clarify the significance of LOH at 19q12 and Xq11–12.
In summary, the present study described for the first time the use of microsatellite analysis to predict the poor outcome for mucinous LMP patients. The results of our study suggest that the identification of LOH at 19q12 and/or Xq11–12 in former mucinous LMP sites should alert the clinician to the presence of a potentially aggressive lesion in the coelomic epithelium, even if the distinction between second primary tumours or recurrence could not be determined.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Beder LB, Gunduz M, Ouchida M, Fukushima K, Gunduz E, Ito S, Sakai A, Nagai N, Nishizaki K, Shimizu K (2003) Genome-wide analyses on loss of heterozygosity in head and neck squamous cell carcinomas. Lab Invest 83: 99–105
Callahan R, Campbell G (1989) Mutations in human breast cancer: an overview. J Natl Cancer Inst 81: 1780–1786
Casey AC, Bell DA, Lage JM, Fuller AF, Nikrui N, Rice LW (1993) Epithelial ovarian tumors of borderline malignancy: long-term follow-up. Gynecol Oncol 50: 316–322
Choi SW, Choi JR, Chung YJ, Kim KM, Rhyu MG (2000) Prognostic implications of microsatellite genotypes in gastric carcinoma. Int J Cancer 89: 378–383
Choi SW, Lee KJ, Bae YA, Min KO, Kwon MS, Kim KM, Rhyu MG (2002) Genetic classification of colorectal cancer based on chromosomal loss and microsatellite instability predicts survival. Clin Cancer Res 8: 2311–2322
Cuatrecasas M, Matias-Guiu X, Prat J (1996) Synchronous mucinous tumors of the appendix and the ovary associated with Pseudomyxoma peritonei. A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol 20: 739–746
Edelson MI, Lau CC, Colitti CV, Welch WR, Bell DA, Berkowitz RS, Mok SC (1998) A one centimorgan deletion unit on chromosome Xq12 is commonly lost in borderline and invasive epithelial ovarian tumors. Oncogene 16: 197–202
FIGO (1987) Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynecology and Obstetrics. Am J Obstet Gynecol 156: 263–264
Gershenson DM (1999) Contemporary treatment of borderline ovarian tumors. Cancer Invest 17: 206–210
Guerrieri C, Franlund B, Boeryd B (1995) Expression of cytokeratin 7 in simultaneous mucinous tumors of the ovary and appendix. Mod Pathol 8: 573–576
Guerrieri C, Franlund B, Fristedt S, Gillooley JF, Boeryd B (1997) Mucinous tumors of the vermiform appendix and ovary, and Pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol 28: 1039–1045
Hata K, Yoshida M, Maruyama R, Fujiwaki R, Miyazaki K (2002) Prognostic significance of ultrasound derived intratumoral peak systolic velocity in epithelial ovarian cancer. Ultrasound Obstet Gynecol 20: 186–191
Hirano A, Emi M, Tsuneizumi M, Utada Y, Yoshimoto M, Kasumi F, Akiyama F, Sakamoto G, Haga S, Kajiwara T, Nakamura Y (2001) Allelic losses of loci at 3p25.1, 8p22, 13q12, 17p13.3, and 22q13 correlate with postoperative recurrence in breast cancer. Clin Cancer Res 7: 876–882
Hu J, Pang JC, Tong CY, Lau B, Yin XL, Poon WS, Jiang CC, Zhou LF, Ng HK (2002) High-resolution genome-wide allelotype analysis identifies loss of chromosome 14q as a recurrent genetic alteration in astrocytic tumours. Br J Cancer 87: 218–224
Huettner PC, Weinberg DS, Lage JM (1992) Assessment of proliferative activity in ovarian neoplasms by flow and static cytometry. Correlation with prognostic features. Am J Pathol 141: 699–706
Kaern J, Trope C, Kjorstad KE, Abeler V, Pettersen EO (1990) Cellular DNA content as a new prognostic tool in patients with borderline tumors of the ovary. Gynecol Oncol 38: 452–457
Knudson AG (1993) Antioncogenes and human cancer. Proc Natl Acad Sci USA 90: 10914–10921
Link Jr CJ, Reed E, Sarosy G, Kohn EC (1996) Borderline ovarian tumors. Am J Med 101: 217–225
Lee KR, Scully RE (2000) Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘Pseudomyxoma peritonei’. Am J Surg Pathol 24: 1447–1464
Mandai M, Konishi I, Kuroda H, Komatsu T, Yamamoto S, Nanbu K, Matsushita K, Fukumoto M, Yamabe H, Mori T (1998) Heterogeneous distribution of K-ras-mutated epithelia in mucinous ovarian tumors with special reference to histopathology. Hum Pathol 29: 34–40
Nakashima N, Nagasaka T, Oiwa N, Nara Y, Fukata S, Fukatsu T, Takeuchi J (1990) Ovarian epithelial tumors of borderline malignancy in Japan. Gynecol Oncol 38: 90–98
Nakayama K, Kanzaki A, Hata K, Katabuchi H, Okamura H, Miyazaki K, Fukumoto M, Takebayashi Y (2002) Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in human ovarian carcinoma. Cancer Lett 176: 215–223
Nakayama K, Takebayashi Y, Nakayama S, Hata K, Fujiwaki R, Fukumoto M, Miyazaki K (2003a) Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett 192: 227–235
Nakayama K, Takebayashi Y, Namiki T, Tamahashi N, Nakayama S, Uchida T, Miyazaki K, Fukumoto M (2001) Comprehensive allelotype study of ovarian tumors of low malignant potential: potential differences in pathways between tumors with and without genetic predisposition to invasive carcinoma. Int J Cancer 94: 605–609
Nakayama S, Nakayama K, Takebayashi Y, Hata K, Fujiwaki R, Fukumoto M, Miyazaki K (2003b) Allelotypes as potential prognostic markers in ovarian carcinoma treated with cisplatin-based chemotherapy. Int J Mol Med 11: 621–625
Nunn J, Scholes AG, Liloglou T, Nagini S, Jones AS, Vaughan ED, Gosney JR, Rogers S, Fear S, Field JK (1999) Fractional allele loss indicates distinct genetic populations in the development of squamous cell carcinoma of the head and neck (SCCHN). Carcinogenesis 20: 2219–2228
Padberg BC, Lauritzen I, Achilles E, Holl K, Bressel M, Kloppel G, Dralle H, Schroder S (1991) DNA cytophotometry in adrenocortical tumours: a clinicomorphological study of 66 cases. Virchows Arch 419: 167–170
Pecorelli S, Fallo L (1998) Hormone replacement therapy in gynecological cancer survivors. Crit Rev Oncol Hematol 27: 1–10
Prayson RA, Hart WR, Petras RE (1994) Pseudomyxoma peritonei. A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol 18: 591–603
Rosin MP, Lam WL, Poh C, Le ND, Li RJ, Zeng T, Priddy R, Zhang L (2002) 3p14 and 9p21 loss is a simple tool for predicting second oral malignancy at previously treated oral cancer sites. Cancer Res 62: 6447–6450
Russel NH, Hunter AE, Haynes A, Bessell EM (1994) Multiple myeloma. BMJ 308: 1715
Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Fearon ER, Hanash SM, Cho KR (2002) Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res 62: 4722–4729
Seidman JD, Elsayed AM, Sobin LH, Tavassoli FA (1993) Association of mucinous tumors of the ovary and appendix. A clinicopathologic study of 25 cases. Am J Surg Pathol 17: 22–34
Serov SF, Scully RE, Sobin LH (1973) Histopathological Typing of Ovarian Tumors. International Classification of Tumors, Vol. 9. Geneva: WHO
Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39: 741–748
Siriaunkgul S, Robbins KM, McGowan L, Silverberg SG (1995) Ovarian mucinous tumors of low malignant potential: a clinicopathologic study of 54 tumors of intestinal and Mullerian type. Int J Gynecol Pathol 14: 198–208
Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM (1999) Molecular genetic evidence supporting the clonality and appendiceal origin of Pseudomyxoma peritonei in women. Am J Pathol 154: 1849–1855
Tamakoshi K, Kikkawa F, Nakashima N, Tamakoshi A, Kawai M, Furuhashi Y, Hattori SE, Kuzuya K, Arii Y, Suganuma N, Tomoda Y (1997) Clinical behavior of borderline ovarian tumors: a study of 150 cases. J Surg Oncol 64: 147–152
Taylor HC (1929) Malignant and semimalignant tumors of the ovary. Surg Obstet Gynecol 48: 204–230
Tazelaar HD, Bostwick DG, Ballon SC, Hendrickson MR, Kempson RL (1985) Conservative treatment of borderline ovarian tumors. Obstet Gynecol 66: 417–422
Trimble CL, Trimble EL (1994) Management of epithelial ovarian tumors of low malignant potential. Gynecol Oncol 55: S52–61
Wingo PA, Tong T, Bolden S (1995) Cancer statistics, 1995. CA Cancer J Clin 45: 8–30
Winter III WE, Kucera PR, Rodgers W, McBroom JW, Olsen C, Maxwell GL (2002) Surgical staging in patients with ovarian tumors of low malignant potential. Obstet Gynecol 100: 671–676
Young RH, Gilks CB, Scully RE (1991) Mucinous tumors of the appendix associated with mucinous tumors of the ovary and Pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol 15: 415–429
Acknowledgements
We thank Dr Tsuneo Namiki and Nobuaki Tamahashi (Institute of Japan Surgical Pathology, Sendai, Japan) for pathological support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Nakayama, K., Takebayashi, Y., Hata, K. et al. Allelic loss at 19q12 and Xq11–12 predict an adverse clinical outcome in patients with mucinous ovarian tumours of low malignant potential. Br J Cancer 90, 1204–1210 (2004). https://doi.org/10.1038/sj.bjc.6601681
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601681
Keywords
This article is cited by
-
High Ki-67 expression is significantly associated with poor prognosis of ovarian cancer patients: evidence from a meta-analysis
Archives of Gynecology and Obstetrics (2019)