Abstract
Data sources
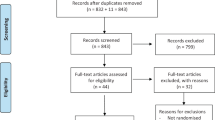
Cochrane CENTRAL Register of Controlled Trials, Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, SCIEXPANDED (ISI Web of Knowledge).
Study selection
Two reviewers selected randomised clinical trials (RCTs) that compared the efficacy of articaine and lidocaine in pain rating during dental treatment in child patients.
Data extraction and synthesis
Two authors extracted data using a standardised form, and risk of bias was assessed based on Cochranes Risk of bias tool for RCTs. Meta-analysis was performed on all included studies (n=6) where self-reported pain during and after dental procedure was recorded, and which compared articaine local anaesthesia (LA) to lidocaine LA in children. Then a sensitive analysis was performed excluding the studies with high overall risk of bias (n=3).
Results
Six studies were included, one had ‘low’, two had ‘moderate/uncertain’ and three had ‘high’ risk of bias. To evaluate the impact of these studies with ‘high’ overall risk of bias, a sensitivity analysis was performed and even when excluding these studies, children who had articaine reported significantly less pain after procedure. However, during the procedure no difference was found between self-reported pain when articaine infiltration and lidocaine inferior dental nerve block were compared.
Conclusions
Low quality evidence suggests no difference in efficacy between lidocaine Inferior alveolar dental nerve blocks and articaine infiltration when used for routine dental treatment in children. Also, no difference was found in self-reported pain between lidocaine and articaine during treatment procedures, but apparently articaine leads to less pain reporting after the procedure. The body of the evidence is quite low due to the substantial heterogeneity in the reported outcomes and the overall high risk of bias of the included studies.
Similar content being viewed by others
Commentary
The most widely used local anaesthesia (LA) solution in dentistry is lidocaine, a fact also observed in Paediatric Dentistry. When treating child patients, it is essential that the anaesthesia is effective, so the child does not experience pain either during or after the entire dental procedure. There is already evidence recommending the use of articaine versus lidocaine for pulpal anaesthesia1 when infiltrative anaesthesia is used, but also as an infiltration supplement during the treatment of mandibular molars with irreversible pulpitis.2 However, the literature lacks high quality evidence concerning which LA solution is more effective in dental treatment for children. The current review aims to assess the efficacy of lidocaine (2%) and articaine (4%), both with epinephrine as a vasoconstrictor, and to compare the outcomes, advantages and harms associated with their use in paediatric dentistry.
The data search was extensively conducted and only randomised control trials (RCTs) involving paediatric population and comparing patient outcomes were included. The primary outcome measure was pain reported, measured during and after the dental procedure, while the secondary outcome measures were adverse events, onset and duration of numbness, need for supplemental injection and vital signs with other physical parameters monitored. All outcomes evaluated were reported using a variety of instruments and the authors used the Hedge's standardised difference in means and corresponding 95% confidence interval to summarise all self-reported outcomes. However, the subjective nature of pain measurement and the methodological inconsistencies and differences in reporting the outcome measures might have resulted in increased heterogeneity of the studies.3 The risk of bias assessment was applied to both study methodology and outcome measure of all included studies. The quality assessment was conducted by two reviewers, independently, following the Cochrane guidelines for assessing risk of bias of RCTs.4 It resulted in only one from the six included studies to be considered as ‘low’ risk of bias, while three studies were at ‘high’ risk of bias.
This suggests that the evidence provided can be considered as low quality. Although no difference was found in self-reported pain during procedure between lidocaine and articaine, Egger's test showed likelihood of publication bias in self-reported pain during procedure (p=0.015). Even though this type of bias is less common in more recent studies,5 it is related to the impediments authors encounter when trying to publish negative results.6 Additionally, in one case in which the study reported the pain score after the injection, the result was categorised as pain score during the procedure, which could have resulted in an under- or overestimation of the effectiveness of the LA solution during the entire dental treatment. Moreover, the authors reported a significant difference in the pain post-procedure favouring the articaine, but this should also be interpreted with caution, as substantial heterogeneity was observed.3 As six studies cannot be considered adequate for subgroup analysis,4 this was not performed, so possible reasons for the heterogeneity were not able to be examined. The fact that various instruments were used in different studies to measure the pain also makes it difficult to carry out a meta-analysis. It calls for more rigorous methodologies including better standardisation of patient outcomes, as adequately addressed by the authors.
References
Brandt RG, Anderson PF, McDonald NJ, Sohn W, Peters MC . The pulpal anesthetic efficacy of articaine versus lidocaine in dentistry: a meta-analysis. J Am Dent Assoc 2011; 142:493–504.
Ashraf H, Kazem M, Dianat O, Noghrehkar F . Efficacy of articaine versus lidocaine in block and infiltration anesthesia administered in teeth with irreversible pulpitis: a retrospective, randomized, double-blind study. J Endod 2013; 39: 6–10.
Ryan R ; Cochrane Consumers and Communication Review Group. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage. Available at http://cccrg.cochrane.org, December 2016. (Accessed October 2018).
Higgins J . Cochrane handbook for systematic reviews of interventions. Available at http://www.cochrane.org/resources/handbook/. (Accessed October 2018).
Kicinski M, Springate DA, Kontopantelis E . Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat Med 2015; 34: 2781–2793. doi:10.1002/sim.6525
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR . Publication bias in clinical research. Lancet 1991; 337: 867–872. doi:10.1016/0140-6736(91)90201-y
Author information
Authors and Affiliations
Additional information
Address for correspondence: Monty Duggal, Faculty of Dentistry, National University of Singapore, 11 Lower Kent Ridge Road, Singapore 119083, Singapore. E-mail: denmasd@nus.edu.sg
Tong HJ, Alzahrani FS, Sim YF, Tahmassebi JF, Duggal M. Anaesthetic efficacy of articaine versus lidocaine in children's dentistry: a systematic review and meta-analysis. Int J Paediatr Dent 2018; 28: 347–360.
Rights and permissions
About this article
Cite this article
Bonifacio, C. The efficacy of articaine and lidocaine local anaesthetic in child patients. Evid Based Dent 19, 105–106 (2018). https://doi.org/10.1038/sj.ebd.6401340
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6401340