Abstract
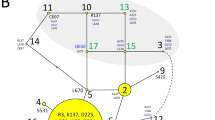
Familial aggregation, high relative risk to siblings, and segregation analysis, suggest genetic control of visceral leishmaniasis in Brazil. Class II gene effects in mice, and high circulating tumour necrosis factor α in humans, provide reasons to target HLA. Fifteen polymorphic markers across 1.03 Mb (DQB1 to TNFa) were genotyped (87 multicase families; 638 individuals). Model-based parametric analyses using single-point combined segregation and linkage in COMDS, or multi-point linkage in ALLEGRO, failed to detect linkage. Model-free nonparametric affected sibling pair (SPLINK) or NPLall score (ALLEGRO) analyses also failed to detect linkage. Information content mapping confirmed sufficient marker information to detect linkage. Analysis of simulated data sets demonstrated that these families had 100% power to detect NPLall scores of 5 to 6 (>LOD4; P < 0.00001) over the range (7% to 61%) of age-related penetrances for a disease susceptibility gene. The extended transmission disequilibrium test (TDT) showed no consistent allelic associations between disease and the 15 loci. TDT also failed to detect significant associations between extended haplotypes and disease, consistent with failure to detect significant linkage disequilibrium across the region. Linkage disequilibrium between adjacent groups of markers (HLADQ/DR; 82–1/82–3/-238bpTNFA; LTA/62/TNFa) was not accompanied by significant global haplotype TDT associations with disease. The data suggest that class II/III regions of HLA do not contain major disease gene(s) for visceral leishmaniasis in Brazil.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ho M, Siongok TK, Lyerly WH, Smith DH . Prevalence and disease spectrum in a new focus of visceral leishmaniasis in Kenya Trans R Soc Trop Med Hyg 1982 76: 741–746
Sacks DL, Lal SL, Shrivastava SN, Blackwell JM, Neva FA . An analysis of T cell responsiveness in Indian Kala-azar J Immunol 1987 138: 908–913
Davies CR, Llanos-Cuentas EA, Pyke SD, Dye C . Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity Epidemiol Infect 1995 114: 297–318
Cabello PH, Lima AM, Azevedo ES, Kriger H . Familial aggregation of Leishmania chagasi infection in northeastern Brazil Am J Trop Med Hyg 1995 52: 364–365
Peacock CS, Collins A, Shaw MA et al. Genetic epidemiology of visceral leishmaniasis in northeastern Brazil Genet Epidemiol 2001 20: 383–396
Blackwell J, Freeman J, Bradley D . Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice Nature 1980 283: 72–74
Blackwell JM . Leishmania donovani infection in heterozygous and recombinant _H-2_ haplotype mice Immunogenetics 1983 18: 101–109
Blackwell JM, Roberts MB . Immunomodulation of murine visceral leishmaniasis by administration of monoclonal anti-Ia antibodies: differential effects of anti-I-A vs anti-I-E antibodies Eur J Immunol 1987 17: 1669–1672
Kaye PM, Cooke A, Lund T, Wattie M, Blackwell JM . Altered course of visceral leishmaniasis in mice expressing transgenic I-E molecules Eur J Immunol 1992 22: 357–364
Barral-Netto M, Badaro R, Barral A et al. Tumor necrosis factor (cachectin) in human visceral leishmaniasis J Infect Dis 1991 163: 853–857
McGuire W, Hill ASV, Allsop CEM, Greenwood BM, Kwiatkowski D . Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria Nature 1994 371: 508–511
Khoo SH, Pepper L, Snowden N et al. Tumour necrosis factor c2 microsatellite allele is associated with the rate of HIV disease progression AIDS 1997 11: 423–428
Roy S, McGuire W, Mascie-Taylor CGN et al. TNF promoter polymorphism and susceptibility to lepromatous leprosy J Infect Dis 1997 176: 530–532
Conway DJ, Holland MJ, Bailey RL et al. Scarring trachoma is associated with polymorphism in the tumor necrosis factor alpha (TNF-alpha) gene promoter and with elevated TNF-alpha levels in tear fluid Infect Immun 1997 65: 1003–1006
McGuire W, Knight JC, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D . Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles J Infect Dis 1999 179: 287–290
Nadel S, Newport MJ, Booy R, Levin M . Variation in the tumor necrosis factor-alpha gene promoter region may be associated with death from meningococcal disease J Infect Dis 1996 174: 878–880
Shaw MA, Donaldson IJ, Collins A et al. Association and linkage of leprosy phenotypes with HLA class II and tumour necrosis factor genes Genes Immun 2001 2: 196–204
Cabrera M, Shaw M-A, Sharples C et al. Polymorphism in TNF genes associated with mucocutaneous leishmaniasis J Exp Med 1995 182: 1259–1264
Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW . Effects of a polymorphism in the human tumour necrosis factor alpha promoter on transcriptional activation Proc NY Acad Sci USA 1997 94: 3195–3199
Louis E, Franchimont D, Piron A et al. Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans Clin Exp Immunol 1998 113: 401–406
Knight JC, Udalova I, Hill AV et al. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria Nat Genet 1999 22: 145–150
Skoog T, van’t Hooft FM, Kallin B et al. A common functional polymorphism (C-->A substitution at position -863) in the promoter region of the tumour necrosis factor-alpha (TNF-alpha) gene associated with reduced circulating levels of TNF-alpha Hum Mol Genet 1999 8: 1443–1449
Singh N, Sundar S, Williams F et al. Molecular typing of HLA class I and class II antigens in Indian kala-azar patients Trop Med Int Health 1997 2: 468–471
Faghiri Z, Tabei SZ, Taheri F . Study of the association of HLA class I antigens with kala-azar Hum Hered 1995 45: 258–261
Meddeb-Garnaoui A, Gritli S, Garbouj S et al. Association analysis of HLA-class II and class III gene polymorphisms in thesusceptibility to mediterranean visceral leishmaniasis Hum Immunol 2001 62: 509–517
Amendoiera R, Guilherme L, Martin M et al. HLA and visceral leishmaniasis in families of endemic area in northeast Brazil Mem I Oswaldo Cruz 1988 83: 119
Blackwell JM, Black GF, Peacock CS et al. Immunogenetics of leishmanial and mycobacterial infections: The Belem Family Study Phil Trans Roy Soc B 1997 352: 1331–1345
Risch N . Assessing the role of HLA-linked and unlinked determinants of disease Am J Hum Genet 1987 40: 1–14
Risch N, Merikangas K . The future of genetic studies of complex human diseases Science 1996 273: 1516–1517
Kemp M, Kurtzhals JAL, Bendtzen K et al. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis Infect Immun 1993 61: 1069–1073
Kurtzhals JA, Hey AS, Jardim A et al. Dichotomy of the human T cell response to Leishmania antigens. II. Absent or Th2-like response to gp63 and Th1-like response to lipophosphoglycan-associated protein in cells from cured visceral leishmaniasis patients Clin Exp Immunol 1994 96: 416–421
Stern JJ, Oca MJ, Rubin BY, Anderson SL, Murray HW . Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis J Immunol 1988 140: 3971–3977
Lainson R, Shaw JJ . Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R (eds) The Leishmaniasis in Biology and Medicine Academic Press: London 1987 1–120
Nascimento MD, Costa JM, Fiori BI et al. The epidemiological determinant aspects in the maintenance of visceral leishmaniasis in the state of Naranhao, Brazil Rev Soc Bras Med Trop 1996 39: 233–240
Grimaldi G, Tesh RB, McMahon-Pratt D . A review of the geographic distribution and epidemiology of leishmaniasis in the New World Am J Trop Med Hyg 1989 41: 687–725
Saiki RK, Bugawan TL, Horn GT, Mullis KB, Erlich HA . Analysis of enzymatically amplified beta-globin and HLA-DQalpha DNA with allele-specific oligonucleotide probes Nature 1986 324: 163–166
Bidwell J . Advances in DNA-based HLA-typing methods Immunol Today 1994 15: 303–307
Kimura A, Sasazuki T . Eleventh International Histocompatibility Workshop reference protocol for the HLA DNA typing technique In: Tsuji K (ed) HLA: OUP 1999 pp 397–419
Lundin KE, Ronningnen KS, Aono S et al. HLA-DQ antigens and DQ beta amino acid 57 of Japanese patients with insulin-dependent diabetes mellitus: detection of a DRw8DQw8 haplotype Tissue Antigens 1989 34: 233–241
Ronningen KS, Iwe T, Halstensen TS, Spurkland A, Thorsby E . The amino acid at position 57 of the HLA-DQ beta chain and susceptibility to develop insulin-dependent diabetes mellitus Hum Immunol 1989 26: 215–225
Hsieh SL, March RE, Khanna A, Cross SJ, Campbell RD . Mapping of 10 novel microsatellites in the MHC class III region: application to the study of autoimmune disease J Rheumatol 1997 24: 220–222
Nedospasov SA, Udalova IA, Kuprash DV, Turetskaya RL . DNA sequence polymorphism at the human tumor necrosis factor (TNF) locus. Numerous TNF/lymphotoxin alleles tagged by two closely linked microsatellites in the upstream region of the lymphotoxin (TNF-β) gene J Immunol 1991 147: 1053–1059
D’Alfonso S, Richiardi PM . A polymorphic variation in a putative regulation box of the TNFA promoter region Immunogenetics 1994 39: 150–154
Wilson AG, di Giovine FS, Blakemore AIF, Duff GW . Single base polymorphism in the human Tumour Necrosis Factor alpha (TNFα) gene detectable by NcoI restriction of PCR product Hum Mol Genet 1993 1: 353
Messer G, Spengler U, Jung MC et al. Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-β gene correlates with a variant amino acid in position 26 and a reduced level of TNF-α production J Exp Med 1991 173: 209–219
Morton NE, Shields DC, Collins A . Genetic epidemiology of complex phenotypes Ann Hum Genet 1991 55: 301–314
Gudbjartsson DF, Jonasson K, Frigge ML, Kong A . Allegro, a new computer program for multipoint linkage analysis Nat Genet 2000 25: 12–13
Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES . Parametric and nonparametric linkage analysis: a unified multipoint approach Am J Hum Genet 1996 58: 1347–1363
Risch N . Linkage strategies for genetically complex traits. II. The power of affected relative pairs Am J Hum Genet 1990 46: 229–241
Risch N . Linkage strategies for genetically complex traits. III. The effect of marker polymorphism on analysis of affected relative pairs Am J Hum Genet 1990 46: 242–253
Holmans P . Asymptotic properties of affected-sib-pair linkage analysis Am J Hum Genet 1993 52: 362–374
Holmans P, Clayton D . Efficiency of typing unaffected relatives in an affected sib-pair linkage study with single locus and multiple tightly-linked markers Am J Hum Genet 1995 37: 1221–1232
Spielman RS, McGinnis RE, Ewens WJ . Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet 1993 52: 506–516
Sham PC, Curtis D . An extended transmission/disequilibrium test (TDT) for multi-allele marker loci Ann Hum Genet 1995 59: 323–336
Falk CT, Rubinstein P . Haplotype relative risks: an easy reliable way to construct a proper control sample for risk calculations Ann Hum Genet 1987 51: 227–233
Dudbridge F, Koeleman BP, Todd JA, Clayton DG . Unbiased application of the transmission disequilibrium test to multilocus haplotypes Am J Hum Genet 2000 66: 2009–2012
Klitz W, Stephens JC, Grote M, Carrington M . Discordant patterns of linkage disequilibrium of the peptide-transporter loci within the HLA class II region Am J Hum Genet 1995 57: 1436–1444
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from The Wellcome Trust, Heather Cordell (CIMR) provided valuable advice on power calculations for linkage. We would also like to thank the people of northeastern Brazil for their hospitality and for their contribution to this study.
Rights and permissions
About this article
Cite this article
Peacock, C., Sanjeevi, C., Shaw, MA. et al. Genetic analysis of multicase families of visceral leishmaniasis in northeastern Brazil: no major role for class II or class III regions of HLA. Genes Immun 3, 350–358 (2002). https://doi.org/10.1038/sj.gene.6363852
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gene.6363852
Keywords
This article is cited by
-
Association between HLA genes and American cutaneous leishmaniasis in endemic regions of Southern Brazil
BMC Infectious Diseases (2013)
-
A longitudinal study on the transmission dynamics of human Leishmania (Leishmania) infantum chagasi infection in Amazonian Brazil, with special reference to its prevalence and incidence
Parasitology Research (2009)
-
Genome-wide scan for visceral leishmaniasis susceptibility genes in Brazil
Genes & Immunity (2007)
-
Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection
Genes & Immunity (2007)
-
Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis
Nature Reviews Genetics (2006)