Abstract
The aim of this study was to explore the role of variants of the gene encoding arachidonate 5-lipoxygenase-activating protein (ALOX5AP) as possible susceptibility factors for coronary artery disease (CAD) and myocardial infarction (MI) in patients with or without angiographically proven CAD. A total of 1431 patients with or without angiographically documented CAD were examined simultaneously for seven ALOX5AP single-nucleotide polymorphisms, allowing reconstruction of the at-risk haplotypes (HapA and HapB) previously identified in the Icelandic and British populations. Using a haplotype-based approach, HapA was not associated with either CAD or MI. On the other hand, HapB and another haplotype within the same region (that we named HapC) were significantly more represented in CAD versus CAD-free patients, and these associations remained significant after adjustment for traditional cardiovascular risk factors by logistic regression (HapB: odds ratio (OR) 1.67, 95% confidence interval (CI) 1.04–2.67; P=0.032; HapC: OR 2.41, 95% CI 1.09–5.32; P=0.030). No difference in haplotype distributions was observed between CAD subjects with or without a previously documented MI. Our angiography-based study suggests a possible modest role of ALOX5AP in the development of the atheroma rather than in its late thrombotic complications such as MI.
Similar content being viewed by others
Introduction
Interest in unraveling the genetic basis of coronary artery disease (CAD) has been recently renewed by results obtained applying powerful approaches such as genome-wide scan studies.1, 2, 3 At variance with classic association studies involving single-nucleotide polymorphisms (SNPs) in candidate genes, genome-wide scan studies have the advantage of discovering new gene(s), without a priori hypothesis. A paradigm of the successful use of such strategies was the identification of arachidonate 5-lipoxygenase-activating protein (ALOX5AP) as a susceptibility gene for myocardial infarction (MI) and stroke.4 Interestingly, ALOX5AP encodes the 5-lipoxygenase-activating protein (FLAP), which is an essential regulator of the biosynthesis of the leukotriene A4 (LTA4).5, 6 Indeed, the 5-lipoxygenase (5-LO)/leukotriene pathway has been independently implicated in the pathogenesis of atherosclerosis in humans7, 8 and mice9 (reviewed by Zhao and Funk10). While not successful in discovering causal variants in ALOX5AP, the original study by Helgadottir et al4 identified a 4-SNP haplotype, named HapA, as a risk factor for MI and stroke in the Icelandic population. The Authors were unable to confirm the result in a cohort of British patients with MI; however, in such cohort they reported an association of another 4-SNP haplotype, named HapB, with MI risk.4 Few subsequent studies on ALOX5AP in different populations yielded conflicting results. Löhmussaar et al11 studied Central European patients with stroke, finding a significant association for several ALOX5AP SNPs, including one that was part of HapA. On the other hand, studies in North Americans failed to show a significant association with either stroke or MI.12, 13
To date, no genetic–epidemiological data are available for populations from Southern Europe. Moreover, none of the previous studies specifically attempted to dissect the role of ALOX5AP in the atherosclerosis phenotype rather than in its ‘complication’ phenotype (MI). We therefore evaluated simultaneously seven ALOXA5 SNPs and their reconstructed haplotypes as possible risk determinants for CAD and MI within the framework of an Italian population with or without angiographically confirmed CAD.
Materials and methods
Study population
The Verona Heart Project is an ongoing study aimed to identify new risk factors for CAD and MI in a population of subjects with angiographic documentation of their coronary vessels. Details about the enrolment criteria have been described elsewhere.14 In the present study, we examined data from a total of 1431 subjects, for whom complete analyses of seven ALOX5AP SNPs (see below) were available. Of these subjects, 1047 had angiographically documented severe coronary atherosclerosis (CAD group), the majority of them being candidates for coronary artery bypass grafting or percutaneous coronary intervention. The disease severity was evaluated by counting the number of major epicardial coronary arteries (left anterior descending, circumflex, and right) affected with ≥1 significant stenosis (≥50%). On the other hand, 384 subjects had completely normal coronary arteries, being submitted to coronary angiography for reasons other than CAD, mainly valvular heart disease (CAD-free group). Controls were also required to have neither history nor clinical or instrumental evidence of atherosclerosis in vascular districts beyond the coronary bed. Since the primary aim of our selection was to provide an objective and clear-cut definition of the atherosclerotic phenotype, subjects with nonsignificant coronary stenosis (ie <50%) were not included in the study. CAD subjects were classified into MI and non-MI subgroups by combining data from history with a thorough review of medical records showing diagnostic electrocardiogram and enzyme changes, and/or the typical sequelae of MI on ventricular angiography. An appropriate documentation was obtained for 1046/1047 (99.9%) CAD patients: from those 624 subjects had a history of previous MI, whereas the remaining 422 subjects had no history of MI. The angiograms were assessed by two cardiologists unaware that the patients were to be included in the study. Samples of venous blood were drawn from each subject after an overnight fast. Serum lipids and the other routine biochemical parameters were determined as described previously.14 At the time of blood sampling, a complete clinical history was collected, including the assessment of cardiovascular risk factors such as obesity, smoking, hypertension, and diabetes.
The study was approved by our local Ethical Committee. Informed consent was obtained from all the patients after a full explanation of the study.
Genotyping
To make possible comparison with studies in other populations, we selected seven previously described ALOX5AP (GeneID: 241; chromosome: 13q12) SNPs (SG13S25, SG13S377, SG13S114, SG13S89, SG13S32, SG13S41 and SG13S35), maintaining their original nomenclature,4 as well as the nomenclature of the reconstructed haplotypes. The seven SNPs were initially tested by PCR and restriction analyses (Supplementary Table 1) in a small group of randomly chosen DNA samples in order to verify the heterozygosity in the study population. All the samples were then genotyped in two multiplex reactions for six SNPs (SG13S377, SG13S41, SG13S32, and SG13S114 in multiplex one, M1; SG13S25, SG13S35 in multiplex two, M2) using LightCycler™ real-time PCR technology based on fluorescence resonance energy transfer and melting point analysis. The sequences of primers and probes used for the six SNPs genotyping with melting point analysis are shown in Supplementary Table 2. Both primers and fluorescently labelled probes were synthesized by Sigma-Proligo (Proligo France SAS). PCR and melting curve analysis was performed in 20 μl volumes in glass capillaries (Hoffmann-La Roche). PCR conditions for M1 and M2 are detailed in Supplementary Tables 3 and 4, respectively. Cycling and melting curve analysis conditions were different for the two multiplex reactions, as given in Supplementary Table 5. As the SG13S89 polymorphism was not easily detectable in a multiplex reaction, it was genotyped by PCR and restriction analysis for all the samples, using the following primers forward (F): 5′-AAGTGCATCTCAAGGAGGT-3′ and reverse (R) 5′-ATTAGCAGAAGAGCCAAGT-3′.
Statistical analysis
The analyses were performed mainly with SSPS 13.0 statistical package (SPSS Inc., Chicago, IL, USA). Distributions of continuous variables in groups were expressed as means±SD. Quantitative data were assessed using the Student's t-test. Associations between qualitative variables were analysed with the χ2 test or Fisher exact test, when indicated. Allele and genotype frequencies among cases and controls were compared with values predicted by Hardy–Weinberg equilibrium using χ2 test. To assess the association with CAD or MI, relative risks associated with each genotype were calculated separately by univariate logistic regression and then by multiple logistic regression adjusted for the traditional cardiovascular risk factors (ie sex, age, smoking, hypertension, diabetes, total cholesterol, and triglycerides), assuming an additive, dominant or recessive mode of inheritance.
Pairwise linkage disequilibrium was examined as described by Devlin and Risch.15 Haplotype frequencies were estimated using R software with haplo.stats package (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0, URL: http://www.R-project.org).16 The upper and lower bounds of the 95% confidence interval (CI) were calculated by simulating 1000 random samples from a population having the haplotype frequencies estimated on the entire sample set. The D′ measure was calculated for each simulated sample. The upper and lower bounds represent the quantiles corresponding to the 0.025 or 0.975 probabilities of the D′ distribution. Haplotype blocks were defined as proposed by Gabriel et al.17 The relationship between haplotypes and clinical outcomes was examined using a generalized linear model regression of a trait on haplotype effects, allowing for ambiguous haplotypes (haplo.glm function),16 and adjusting for the above-mentioned risk factors. Randomization test by permuting the cases and controls was performed by means of Monte Carlo method to confirm the results. Haplotypes present in less than 10 individuals were not considered in the analyses.
The study power was assessed by means of the Altman nomogram, after adjustment for the asymmetric distribution of population subgroups (CAD-free versus CAD; non-MI versus MI). The study has adequate power (>90%) to replicate the findings for odds ratios (ORs) greater than 2.0, which is consistent with those observed in the previous studies.4 For each OR, 95% CIs were calculated. A value of two-tailed P<0.05 was considered significant.
Results
Table 1a summarizes the clinical characteristics of the study population stratified according to the presence (CAD) or absence (CAD-free) of angiographically documented CAD. As expected, traditional cardiovascular risk factors were more represented in the CAD group. The characteristics of the CAD population, divided in two subgroups according to the presence or absence of a previous documented MI, are reported in Table 1b. The genotype frequencies for the polymorphisms tested were in Hardy–Weinberg equilibrium both in the CAD and CAD-free groups.
Allele and genotype distributions were similar either between CAD and CAD-free groups (Table 2a) or within CAD subjects with or without a previous MI (Table 2b). Results from the regression analyses, assuming additive, dominant or recessive mode of inheritance, showed no significant association of the gene variants tested with the clinical outcomes (data not shown). In general, the SNPs tested were in linkage disequilibrium, as shown in Table 3.
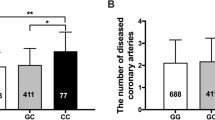
Considering haplotype analysis, the most frequent haplotypes were G-T-G-C and G-T-A-G for HapA SNPs and HapB/C SNPs, respectively, and thus were used as the referents. The haplotype distributions for HapA SNPs were similar between CAD and CAD-free subjects (P=0.937). On the other hand, the haplotype distributions for HapB SNPs were significantly different between CAD and CAD-free subjects (P=0.014), as shown in Table 4a. More precisely, two haplotypes A-A-A-G (HapB) and G-T-A-A (that we named HapC) were more represented in CAD group (7.5 versus 5.5% and 3.7 versus 1.6%, respectively), and these associations remained significant also after adjustment for traditional cardiovascular risk factors, that is, sex, age, smoking, hypertension, diabetes, total cholesterol, and triglycerides (Table 4b). The significance of the general model, including genetic factors arranged as haplotypes, was confirmed after randomization test (P=0.022 for general model, P=0.013 for HapB and P=0.021 for HapC, after 1000 permutations).
There was no difference in haplotype distributions between CAD subjects with or without a previous MI, either for HapA region or for HapB region (Table 4c).
Discussion
The present investigation in Italian patients provides some evidence that the ALOX5AP gene might play a role in conferring susceptibility to CAD also in South European populations. Nonetheless, since the statistically significant association we found was relatively weak, the role of this gene, if any, seems modest. To put our results into a more general perspective, we propose the following considerations.
Comparison with previous studies
The landmark study by Helgadottir et al identified two different haplotypes as CAD risk factors in populations of different ancestry. According to the haplotype block definition proposed by Gabriel et al,17 we observed three haplotype blocks of two SNPs each (block 1: SG13S25-SG12S377; block 2: SG13S32-SG13S41; block 3: SG13S41-SG13S35). Therefore, the SNPs describing HapA or HapB/C do not define a single haplotype block. This finding is consistent with what observed by Helgadottir et al.4
In Icelandics (a genetic isolate), the HapA conferred a nearly twofold risk of MI.4 This was not confirmed in British patients, in whom, on the other hand, a different 4-SNPs haplotype (HapB) was associated with MI. Neither HapA nor HapB was associated to incident MI in a cohort of male US physicians.13 To make possible a comparison, we focused on a standardized set of seven ALOX5AP SNPs, allowing reconstruction of the same at-risk haplotypes reported in the literature. With respect to HapA, our results suggest that this haplotype may not be informative for risk assessment of CAD in non-Icelandic populations. With respect to HapB, a modest contribution of this genetic marker to CAD risk was observed. Haplotype analyses revealed in our population a nominally significant association between CAD and another ALOX5AP haplotype (‘HapC’), unremarkable in previous studies. Considering also the low frequency of this haplotype, the relevance of this finding remains uncertain. The observed differences among populations are not surprising, and may relate in part to population-specific differences in allele and haplotype frequencies (for a summary of previous studies see Table 5). For example, the frequency of HapA in Icelandic controls (9.5%) was well below that observed in North American (15%), German (15.2%), and our Italian (18.6%) populations. Moreover, it has to be underscored that we are dealing with disease-risk-associated haplotypes made of SNPs with no obvious potential effects on function, whose association(s) with yet unidentified causal variant(s) in ALOX5AP may differ between populations with differing genealogies. In other words, it would not be unexpected to find in the future different pathogenic ALOX5AP mutation(s), with different frequencies among populations, arising on different haplotype background. Noteworthy, a replication study in a Japanese population18 found an allele frequency of HapA/HapB SNPs too low to conduct meaningful association. Nevertheless, in that population haplotypes constructed on the basis of two other intronic SNPs were significantly associated with MI.
ALOX5AP, leukotriene pathway, and CAD pathogenesis
Preliminary functional data by Helgadottir et al indicated that some at-risk haplotypes were associated to increased neutrophil release of leukotriene B4 (LTB4). Being LTB4 synthesized from LTA4, it implies that ALOX5AP variants might determine proinflammatory gain of functions. The role of inflammation in CAD pathogenesis is now well-established (reviewed by Hansson19). The FLAP protein encoded by ALOX5AP has an important role in the initial steps of the biosynthesis of leukotrienes,5, 6 which in turn have a variety of proinflammatory effects.20 Besides the ALOX5AP story, genetic evidence for the involvement of the 5-LO/leukotriene pathway in CAD is accumulating.21, 22, 23 The same Icelandic group recently reported that another gene involved in this pathway, that is, leukotriene A4 hydrolase, conferred risk of CAD, especially in African Americans.21 Dwyer et al22 found an association between promoter variations of the ALOX5 gene (encoding 5-LO, ie the FLAP target) and carotid intima-media thickness (a preclinical surrogate marker of atherosclerosis). As a functional counterpart of intriguing genetic studies, a bulk of animal experiments have linked the 5-LO pathway to atherosclerosis, although results are sometimes discordant (critically reviewed by Funk23). Interestingly, many of basic researches leading to the ‘lipoxygenases hypothesis’24, 25 points towards an involvement in early events of atheroma development, through LTB4-mediated migration and activation of monocyte/macrophages, as well as lipoxygenases-mediated LDL oxidation.
Peculiarities of the present study: strengths and limitations
Previous studies on ALOX5AP focused on MI patients versus controls selected from the general population or from event-free subjects such as in the prospective Physician's Health Study cohort.4, 13 MI is usually a late thrombotic complication superimposed on coronary atherosclerotic plaque rupture,26 so that design of previous studies did not directly allow to separate a putative specific role of ALOX5AP in MI rather than in CAD development. Our alternative experimental design focused on subjects with angiographically proven CAD, with or without a previous documented MI. Moreover, the angiography-based design enabled us to select CAD-free subjects with an objectively defined control status, a critical issue in genetic association studies.27 This allowed us to overcome the caveat, common in Western general populations where atherosclerosis is endemic, of enrolling controls with substantial coronary atherosclerotic lesions, although not yet clinically evident. While our CAD-free subjects cannot be considered a ‘typical’ control group, we feel confident about their acceptable representativity of the background general population, being their genotype and haplotype distributions, not fundamentally different from those observed in controls from German and US populations (see above). Since we noted haplotype differences only between the whole CAD group versus the CAD-free group, and not between CAD patients with or without MI, our data appear to be consistent with a more relevant role of ALOX5AP in atherogenesis rather than in thrombogenesis, according to many of the above-mentioned biochemical data.
This study suffers of common limitations of genetic association studies with complex traits.28 Despite the unbalance between case and controls, it was sufficiently powered to detect a predefined effect of ALOX5AP haplotypes on CAD (see above). On the other hand, we could not properly analyse some interesting issues such as a possible stronger effect of ALOX5AP in males than in females,4 because of the limited number of women enrolled.
Finally, from a possible practical perspective it has to be taken into account the relatively poor frequency of ‘at-risk’ haplotypes in our population, as well as their modest effect on the CAD risk.
Conclusions
ALOX5AP represents the paradigm of a new class of promising genes identified by powerful genome-wide investigations, which is currently an object of intense investigations to confirm their role in CAD susceptibility. Our data neither refute nor strongly support this hypothesis. Adding them to current knowledge, some evidence on ALOX5AP as a genetic susceptibility factor for CAD has now emerged in four out of five independent populations (Icelandic, British, Japanese, and Italian; but not in North America). Our angiography-based study suggests a possible role of ALOX5AP/FLAP in the development of the atheroma rather than in its late thrombotic complications such as MI. Such a role, if any, appears to be modest. Much further work is needed to understand the reason(s) for heterogeneous results, as well as to identify possible ALOX5AP pathogenic variations.
References
Watkins H, Farrall M : Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet 2006; 7: 163–173.
Lusis AJ, Fogelman AM, Fonarow GC : Genetic basis of atherosclerosis, Part I, New genes and pathways. Circulation 2004; 110: 1868–1873.
Wang Q : Molecular genetics of coronary artery disease. Curr Opin Cardiol 2005; 20: 182–188.
Helgadottir A, Manolescu A, Thorleifsson G et al: The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 2004; 36: 233–239.
Dixon RAF, Diehl RE, Opas E et al: Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 1990; 343: 282–284.
Miller DK, Gillard JW, Vickers PJ et al: Identification and isolation of a membrane protein necessary for leukotriene production. Nature 1990; 343: 278–281.
Spanbroek R, Grabner R, Lotzer K et al: Expanding expression of 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA 2003; 100: 1238–1243.
Qiu H, Gabrielsen A, Agardh HE et al: Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci USA 2006; 103: 8161–8166.
Mehrabian M, Allayee H, Wong J et al: Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res 2002; 91: 120–126.
Zhao L, Funk CD : Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med 2004; 14: 191–195.
Löhmussaar E, Gschwendtner A, Mueller JC et al: ALOX5AP gene and the PDE4D gene in a central European population of stroke patients. Stroke 2005; 36: 731–736.
Meschia JF, Brott TG, Brown RD et al: Phosphodiesterase 4D and 5-lipoxygenase activating protein in ischemic stroke. Ann Neurol 2005; 58: 351–361.
Zee RY, Cheng S, Hegener HH, Erlich HA, Ridker PM : Genetic variants of arachidonate 5-lipoxygenase activating protein and risk of incident myocardial infarction and ischemic stroke. Stroke 2006; 37: 2007–2011.
Girelli D, Russo C, Ferraresi P et al: Polymorphisms in the factor VII gene and the risk of myocardial infarction in patients with coronary artery disease. N Engl J Med 2000; 343: 774–780.
Devlin B, Risch N : A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics 1995; 29: 311–322.
Lake SL, Lyon H, Tantisira K et al: Estimation and tests of haplotype–environment interaction when linkage phase is ambiguous. Hum Hered 2003; 55: 56–65.
Gabriel SB, Schaffner SF, Nguyen H et al: The structure of haplotype blocks in the human genome. Science 2002; 296: 2225–2229.
Kajimoto K, Shioji K, Ishida C et al: Validation of the association between the gene encoding 5-lipoxygenase-activating protein and myocardial infarction in a Japanese population. Circ J 2005; 69: 1029–1034.
Hansson GK : Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695.
Samuelsson B : Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 1983; 220: 568–575.
Helgadottir A, Manolescu A, Helgason A et al: A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet 2006; 38: 68–74.
Dwyer JH, Allayee H, Dwyer KM et al: Arachidonate 5-lipoxigenase promoter genotype, dietary arachidonic acid and atherosclerosis. N Engl J Med 2004; 350: 29–37.
Funk CD : Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov 2005; 4: 664–672.
Steinberg D : At last, direct evidence that lipoxygenases play a role in atherogenesis. J Clin Invest 1999; 103: 1487–1488.
Lötzer K, Funk CD, Habenicht AJR : The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta 2005; 1736: 30–37.
Lusis AJ : Atherosclerosis. Nature 2000; 407: 233–241.
Lander ES, Schork NJ : Genetic dissection of complex traits. Science 1994; 265: 2037–2048.
Colhoun HM, McKeigue PM, Davey Smith G : Problems of reporting genetic associations with complex outcomes. Lancet 2003; 361: 865–872.
Helgadottir A, Gretarsdottir S, St Clair D et al: Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet 2005; 76: 505–509.
Acknowledgements
This work was supported by grants from the Veneto Region, Italian Ministry of University and Research (Grant no. 2005/065152), and the Cariverona Foundation, Verona, Italy. We wish to thank Mrs Maria Zoppi for invaluable secretary help, and Mr Diego Minguzzi for technical assistance. The authors declare that they have no potential conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Girelli, D., Martinelli, N., Trabetti, E. et al. ALOX5AP gene variants and risk of coronary artery disease: an angiography-based study. Eur J Hum Genet 15, 959–966 (2007). https://doi.org/10.1038/sj.ejhg.5201854
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201854
Keywords
This article is cited by
-
Genetic variation in the leukotriene pathway is associated with myocardial infarction in the Chinese population
Lipids in Health and Disease (2019)
-
Weighted Multi-marker Genetic Risk Scores for Incident Coronary Heart Disease among Individuals of African, Latino and East-Asian Ancestry
Scientific Reports (2018)
-
The rs1803274 polymorphism of the BCHE gene is associated with an increased risk of coronary in-stent restenosis
BMC Cardiovascular Disorders (2015)
-
A Novel Risk Haplotype of ALOX5AP Gene is Associated with Ischemic Stroke in Chinese Han Population
Journal of Molecular Neuroscience (2014)
-
ALOX5AP gene variants show differential association with coronary artery disease in different populations
Journal of Community Genetics (2010)