Abstract
To investigate the genetic influence on X chromosome inactivation and on age-related skewing of X inactivation, in particular, we analysed the X inactivation pattern (XIP) in peripheral blood cells from 118 young monozygotic (MZ) twin pairs (18–53 years), 82 elderly MZ twin pairs (55–94 years), 146 young dizygotic (DZ) twin pairs (20–54 years) and 112 elderly DZ twin pairs (64–95 years). Elderly twins had a higher frequency of skewed X inactivation (34%) than young twins (15%) (P<0.001). Our data suggest that the increase in skewing occurs after age 50–60 years. The intraclass correlation was 0.61 and 0.58 in young and elderly MZ twin pairs, and 0.08 and 0.09 in young and elderly DZ twin pairs. Biometric analysis showed that dominant genetic effects accounted for 63 and 58% of the variance of XIP in the young and elderly twin pairs, respectively. The dominant genetic effect and the shared environment for monochorionic MZ twins may explain the high intraclass correlation for the MZ twin pairs compared to the DZ twin pairs. We did not observe a significant decrease in the intraclass correlation in elderly MZ twins compared to young MZ twins, which would be expected if age-related skewing were due to stochastic factors. We conclude that the increased skewing with age implies that a genetically dependent selection of blood cells take place.
Similar content being viewed by others
Introduction
In female mammalian cells, one of the two X chromosomes is inactivated in early embryonic life.1 Females are therefore mosaics for two cell lines, one with the maternal X as the active X chromosome, and one with the paternal X as the active X chromosome. In young females, the distribution of the two cell lines is close to normal with a mode corresponding to the 50:50 ratio. A skewed X inactivation is a marked deviation from the 50:50 ratio and may be primary, either due to chance or to factors that affect the process of X inactivation during early embryo development.2, 3 A skewed X inactivation may also be secondary due to a selection process against or in favour of cells with a specific genotype.4 Although X inactivation has been assumed to be random for each cell, and permanent for all descendants of the cell, there is evidence that the pattern of X inactivation is also genetically controlled.5, 6, 7, 8, 9, 10
Elderly females have a much higher frequency of skewed X inactivation in the myeloid cell lineage of peripheral blood cells compared to younger females.11 This age-related skewing of X inactivation could be the result of a clonal stochastic loss of haematopoietic cells12, 13, 14, 15 or to competitive advantage for haematopoietic stem cells with a specific genotype of X-linked genes, as suggested by Abkowitz et al.16
In a study of DNA from peripheral blood cells from 71 elderly monozygotic (MZ) twin pairs (73–93 years), we found a strong tendency for the same X chromosome in a twin pair to be the predominating active X chromosome, and an intraclass correlation coefficient of 0.57 for X inactivation pattern (XIP).9 This observation is consistent with an effect of X-linked genes on the X inactivation phenotype in elderly females. To determine if the correlation within twin pairs increased (in case of a strong genetic influence on age-related skewing) or decreased (in case of stochastic processes) with age, we analysed the XIP in 118 young MZ twin pairs, and in 146 young and 112 elderly dizygotic (DZ) twin pairs, for comparison.
Subjects and methods
Twin material
Blood samples were obtained from MZ and DZ twin pairs in The Longitudinal Study of Aging Danish Twins in the Nationwide Danish Twin Registry17, 18, 19 and The Twin Study of The Metabolic Syndrome (GEMINAKAR)20 after informed consent. Zygosity was determined using a PCR analysis of the highly polymorphic markers D7S482, ApoB, D19S19, LIPE and the androgen receptor (AR), or by means of nine polymorphic DNA-based microsatellite markers with the PE Applied Biosystems AmpFISTR Profiler Plus Kit.
Blood samples were available from 310 DZ twin pairs aged 20–95 years, and 151 MZ twin pairs aged 18–73 years. Of these, 258 DZ pairs and 129 MZ pairs were informative for X inactivation analysis. In addition, the 71 previously analysed elderly MZ twin pairs9 were included, giving a total number of 200 informative MZ twin pairs.
Singletons
In order to compare the XIP in various age groups, blood samples from 93–95-year-old females from the Danish 1905 Cohort21 were included. Of these, 80 were informative for X inactivation analysis.
Also included were 144 previously analysed premenopausal females aged 20–44 years and 91 postmenopausal females aged 55–72 years,22 43 females aged 83–101 years23 and 33–101–year-old females.9
X inactivation analysis
XIP was determined by PCR analysis of a polymorphic (CAG)n repeat in the first exon of the androgen receptor (AR) gene.24 After digestion of DNA with the methylation-sensitive enzyme HpaII, a PCR product is obtained from the inactive X chromosome only. The PCR products were separated on an ABI 373 or ABI 3100 automated sequencer, and analysed by GeneScan software (Applied Biosystems). Each sample was analysed in duplicate and without knowledge of the results from the co-twin.
We used two measures of X inactivation. Degree of skewing (DS) describes the magnitude of skewing. DS designates the percentage of the most intense PCR product (the preferentially inactive allele). DS varies between 50 and 100, where 50 indicates a random X inactivation pattern and 100 a completely skewed inactivation pattern. XIP describes both the magnitude and the direction of skewing. For DZ twin pairs who had similar AR alleles, the XIP was recorded as the percentage of the PCR product of the smallest AR allele. For DZ twin pairs who differed in one allele in the AR locus, the parental origin of the X chromosome could be identified, and the XIP was recorded as the percentage of the amount of PCR product of the paternally inherited X chromosome instead of the smallest allele. XIP varies between 0 and 100, where 50 is a random, and 0 and 100 is a completely skewed X inactivation.
XIP was arbitrarily classified as skewed when 80% or more of the cells preferentially inactivated one X chromosome.
Statistical methods
The relationship between age and DS was illustrated using the Lowess procedure, which draws a smooth curve through data using a robust regression.25 Since a relationship between MZ twinning and X inactivation has been suggested,26, 27, 28 MZ twins were excluded from this analysis. For the DZ twins, only one randomly chosen twin was included from each pair. The Pearson correlation coefficient was calculated to test the association between age and DS.
Pearson's χ2 was used for the comparison of frequencies. P-values ⩽0.05 was set as statistical significant.
The intraclass correlation coefficient was calculated for twin pairs and was determined as twice the covariance between twins in a pair, divided by the sum of the variance for each twin in a pair.
Structural equation modelling
Structural equation modelling (SEM) was used to provide estimates of genetic and environmental components of variance of X inactivation.29 The genetic contribution was divided into variance due to additive (A) genetic effects and variance due to dominance (D). The environmental contribution was divided into variance due to shared environmental effects (C) and variance due to unique environmental effects (E). This latter component also includes measurement error. The study design only allows estimation of a maximum of three components in the same model. The interpretation of the variance components is based on a variety of assumptions.29 Particularly important is the so-called ‘equal environment assumption’ implying that the common environmental effect (C) is equal for MZ and DZ twins. The significance of the components A, D, C and E were tested by removing them sequentially in submodels and comparing them with the full models. The selection of the best fitting model was based on a balance between goodness of fit and parsimony. The fit of models were tested by χ2 tests, where a small χ2-value and a high P-value indicated a good fit between the model and the observed data. Parsimony was assessed by means of Akaike's Information Criterion (AIC), which corresponds to the χ2 value minus 2 times the number of degrees of freedom.30 A negative AIC indicated the best fitting models. The software Mx was used for these analyses.31
Results
Age and DS
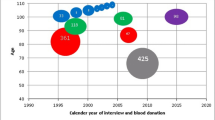
The relationship between age and DS was analysed using the data from all singletons and one randomly chosen DZ twin from each pair. The correlation between age and DS was 0.31 (P<0.001) (Figure 1). The Lowess curve25 detected an increase of skewing after age 50–60 years (Figure 1).
Scatterplot of age and DS in DZ twins (one twin from each pair, randomly selected), 91 postmenopausal females, eighty 95-year-old females and 33 centenarians. In all, 50 indicates random and 100 a completely skewed XIP. A Lowess curve between age and the degree of skewing suggests that the degree of skewing start to increase around age 50–60 years.
Age and frequencies of skewed X inactivation
The total twin population was divided into young twins (<55 years) and elderly twins (≥55 years). The frequency of skewed X inactivation in young MZ twins (12%) was slightly lower than that of young DZ twins (17%) (χ2=2.53, P=0.11) (Figure 2a), whereas the frequency of skewed X inactivation in elderly MZ twins (33%) did not differ from that of DZ twins of the same age group (35%) (χ2=0.23, P=0.63). The frequency of skewed X inactivation in the elderly twins was much higher than the frequency in the young twins (15%) (χ2=49.10, P<0.001). Furthermore, the frequency of skewed X inactivation also in the centenarians (67%) was significantly higher than in 95-year-old females (45%) (χ2=4.39, P=0.04) (Figure 2b).
Analysis of twin similarity
There was a significant intraclass correlation of DS for both young and elderly MZ twin pairs (0.67 and 0.51, respectively). Young and elderly DZ twin pairs were also correlated (0.18 and 0.22, respectively), suggesting that sisters are more similar with respect to X inactivation pattern than unrelated females.
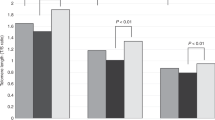
The intraclass correlation using DS considers the magnitude of skewing and is expected to be responsive to any genetic factor that affects skewing per se. The intraclass correlation using XIP also takes into account the direction of skewing and is expected to respond to any genetic factor that have a preference for one of the two X chromosomes. The intraclass correlations of XIP are illustrated in Figure 3. There was a significant intraclass correlation of XIP for both young and elderly MZ twin pairs (0.61 and 0.58, respectively) but not for young or elderly DZ twin pairs (0.08 and 0.09, respectively) according to the confidence intervals (Table 1). There was a significant difference between MZ and DZ twin pairs in both age groups, suggesting a genetic influence on the XIP.
Since the intraclass correlations using XIP show both the magnitude of X inactivation skewing and the direction of skewing, the genetic contribution to X inactivation was measured using the XIP results. The best fitting model was one including only D and E according to the criteria described in the Subjects and methods section, with a heritability of 0.61 (Table 2a). Biologically, it is rare to have genetic dominance in the absence of genetic additivity, and the outcome of the analysis raises the suspicion that the equal environment assumption is not fulfilled. However, the measure of XIP is relative (strength of one X versus another), which may explain the absence of genetic additivity for this particular trait.
When the twin group was divided into younger twins (<55 years) and elderly twins (≥55 years), a DE model also provided the best fit to the data (Table 2b). According to this model, the heritability of XIP was 0.63 and 0.58 for young and elderly twins, respectively.
Parental origin of preferentially active X chromosome
There is no evidence of imprinting of X inactivation in somatic cells in the human.32, 33 However, to exclude an imprinting effect in the age-related skewing of X inactivation, we determined the parental origin of the predominantly active X chromosome both in the young and in the elderly DZ twins. Of the younger twins, 10 of 18 (55%) had the maternal X and 8 of 18 (45%) had the paternal X as the predominating active X chromosome. Similar results were found in elderly DZ twins, where 15 of 29 (52%) of twins had the maternal X and 14 of 29 (48%) of twins had the paternal X as the active X chromosome.
Discussion
No increased frequency of skewed X inactivation in MZ twins
X inactivation occurs at late blastocyst stage (∼5 days after fertilization),34, 35 which is about the same time as the MZ twinning process.36 A relationship between MZ twinning and X inactivation has therefore been suggested, with an expected higher frequency of skewed X inactivation in MZ twins.26, 27 In a study of umbilical cord tissue in 43 MZ and 24 DZ twin pairs, a higher frequency of skewed X inactivation was found in MZ twins compared to DZ twins.37 This increase was not confirmed in the present study, since we found that young MZ twins had a slightly lower frequency of skewed X inactivation than young DZ twins (P=0.11), and did not differ from young singletons (P=0.44). This is also in agreement with the results of Monteiro et al,28 who found the XIP in young MZ twins to be similar to young singletons. Furthermore, also in the elderly MZ twins we found the frequency of skewed X inactivation to be similar to that of elderly DZ twins and elderly singletons.
Genetics of X inactivation
Genetic control of X inactivation has been demonstrated in mice, where the choice of which X chromosome to be inactivated is under the control of the X-controlling element (Xce).38 This locus contains alleles of different ‘strength’. In a female mouse heterozygous for a ‘strong’ and a ‘weak’ allele, the X chromosome that carries the ‘strong’ Xce allele is more likely to be active than the X chromosome that carries the ‘weak’ allele. These mice have a 20–30% distortion from the 50:50 distribution of XIP.39, 40 Furthermore, mouse studies have recently revealed two autosomal dominant mutations that affect X chromosome choice early in development causing skewed XIP.10
In humans, selection for an advantage in proliferation of peripheral blood cells has been seen in female carriers of several X-linked disorders.3, 41 However, the increased skewing of X inactivation in phenotypically normal females has been more puzzling. Mutations in the minimal promoter of the XIST (X inactivation specific transcript) gene have been found in families with familial skewed X inactivation.7, 42 However, these mutations are rare, and do not explain other reports of familial occurrence of skewed X inactivation.2, 6, 43, 44 Linkage analysis has mapped X inactivation phenotype in 38 normal families to the region of the X inactivation centre and to Xq25–26.8
There are few studies on the XIP in twins. MZ twins may be monochorionic, where the two embryos have a common placenta and chorion and therefore share a common blood supply, or dichorionic where each embryo has its own placenta and chorion. About 1/3 of MZ twins are dichorionic and result from a twinning event that occurs about 0–4 days after conception. The remaining 2/3 of MZ twins are monochorionic, and seem to be the result of an event that occurs >4 days after fertilization.45, 46 A highly similar XIP has been reported for monochorionic but not for dichorionic twin pairs.28, 47 It was suggested that monochorionic twin pairs undergo splitting after X inactivation has taken place, while dichorionic twin pairs split before or around the time of X inactivation. In our study, as in most twin studies, information on chorion and placenta was not available. We found an intraclass correlation of XIP, which was much higher for the young MZ twin pairs (0.61) than for the young DZ twin pairs (0.08). The heritability was estimated to be 0.63 and a role of dominant genes is suggested. Part of the high correlation for XIP in the young MZ twin pairs may be due to gene interactions, which would add to the genetic dominance, or due to similarity in the monochorionic twins, and not related to genetic factors. However, since DS for young DZ twin pairs is correlated, a genetic influence on the XIP is likely. A human homologue of the mouse Xce locus, with different ‘strengths’ that influence the XIP in humans would give rise to several different X inactivation phenotypes. Such a locus is in accordance with a dominance model and could explain the many DZ twin pairs with opposite XIP found in this study and, again, the low intraclass correlation of XIP for DZ twin pairs.
Age-related skewing of X inactivation
An acquired skewing with increased age has been reported to occur mainly in blood cells of the myeloid cell lineage.14, 48 We could confirm an increased frequency of a skewed XIP in elderly females. This increase seemed to continue throughout life, also for females between 90 and 100 years. In a recent study of 350 females aged 0–88 years, a significant correlation was found between age and DS (r=0.23, P<0.0001),49 which is in agreement with our results. They also found a tendency of an increase in skewing after a certain age as we have seen in this study. The reasons for age-related skewing are not known. However, our findings of an increase after 50–60 years could indicate that hormonal changes following menopause may contribute to age-related skewing.
In a study of X inactivation in granulocytes from 29 MZ pairs and 18 DZ pairs aged 51–73 years, an intraclass correlation for XIP for elderly MZ twin pairs was found to be 0.53, which is in agreement with our results.50 The intraclass correlation of XIP in their elderly DZ twin pairs was 0.19, also much lower than in their MZ twins.
The genetic contribution to the X inactivation phenotype in the elderly twins in our study was suggested to be dominant with an estimated heritability of 0.58. The weak, but significant intraclass correlation of DS for the elderly DZ twin pairs support this assumption. However, the population of skewed elderly twins is a mixture of twins who have been skewed since they were young, and twins who have an acquired age-related skewing. Therefore, as for the young MZ twin pairs, part of the high intraclass correlation for elderly MZ twin pairs could be an effect of similar XIP within monochorionic MZ twin pairs. The frequency of skewed X inactivation increased from 12% in the young MZ twin pairs to 33% in the elderly MZ twin pairs. If the age-related skewing was a stochastic phenomenon, and therefore random, it would be expected that the correlation for MZ twin pairs and the heritability amongst twins would decrease with increasing age. Conversely, if age-related skewing was due predominantly to selective differences between the two X chromosomes, the correlation should increase with age. Since this was not the case, we conclude that the age-related skewing is a combination of stochastic and genetic events.
The consequences of the age-related skewing of X inactivation are not known. One possible consequence of skewing with advanced age is the manifestation of X-linked disorders in elderly carrier females. There are reports of elderly female carriers with X-linked haematopoietic disorders who showed late manifestation of the disease caused by unfavourable skewed X inactivation.51, 52 Manifestations of an X-linked disorder in elderly females should therefore be considered as a possibility in other X-linked haematopoietic disorders such as X-linked hyper-IgM syndrome, severe combined immunodeficiency and chronic granulomatous disease.
In summary, we found evidence of a genetic influence on the X inactivation phenotype, which is in agreement with previous family studies.8 We have showed that aging leads to more variation in the X inactivation phenotype and that the increase of skewing seems to continue throughout life. Our data indicate that age-related skewing is not solely a stochastic process and may be due to complex mechanisms that involve both stochastic and genetic events.
We have studied X inactivation in blood cells, a mixture of cells that may be differently influenced by aging. Family studies of X inactivation in elderly females where DNA is available from various cell types may increase the knowledge of the effect of genetic factors on age-related skewing.
References
Lyon MF : Gene action in the X-chromosome of the mouse (Mus musculus L). Nature 1961; 190: 372–373.
Belmont JW : Genetic control of X inactivation and processes leading to X-inactivation skewing. Am J Hum Genet 1996; 58: 1101–1108.
Van den Veyver IB : Skewed X inactivation in X-linked disorders. Semin Reprod Med 2001; 19: 183–191.
Migeon BR, Haisley-Royster C : Familial skewed X inactivation and X-linked mutations: unbalanced X inactivation is a powerful means to ascertain X-linked genes that affect cell proliferation. Am J Hum Genet 1998; 62: 1555–1557; discussion 1557–1558.
Cattanach BM, Perez JN, Pollard CE : Controlling elements in the mouse X-chromosome. II. Location in the linkage map. Genet Res 1970; 15: 183–195.
Naumova AK, Plenge RM, Bird LM et al: Heritability of X chromosome – inactivation phenotype in a large family. Am J Hum Genet 1996; 58: 1111–1119.
Plenge RM, Hendrich BD, Schwartz C et al: A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet 1997; 17: 353–356.
Naumova AK, Olien L, Bird LM et al: Genetic mapping of X-linked loci involved in skewing of X chromosome inactivation in the human. Eur J Hum Genet 1998; 6: 552–562.
Christensen K, Kristiansen M, Hagen-Larsen H et al: X-linked genetic factors regulate hematopoietic stem-cell kinetics in females. Blood 2000; 95: 2449–2451.
Percec I, Plenge RM, Nadeau JH, Bartolomei MS, Willard HF : Autosomal dominant mutations affecting X inactivation choice in the mouse. Science 2002; 296: 1136–1139.
Busque L, Mio R, Mattioli J et al: Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 1996; 88: 59–65.
Abkowitz JL, Catlin SN, Guttorp P : Evidence that hematopoiesis may be a stochastic process in vivo. Nat Med 1996; 2: 190–197.
Champion KM, Gilbert JG, Asimakopoulos FA, Hinshelwood S, Green AR : Clonal haemopoiesis in normal elderly women: implications for the myeloproliferative disorders and myelodysplastic syndromes. Br J Haematol 1997; 97: 920–926.
Gale RE, Fielding AK, Harrison CN, Linch DC : Acquired skewing of X-chromosome inactivation patterns in myeloid cells of the elderly suggests stochastic clonal loss with age. Br J Haematol 1997; 98: 512–519.
Tonon L, Bergamaschi G, Dellavecchia C et al: Unbalanced X-chromosome inactivation in haemopoietic cells from normal women. Br J Haematol 1998; 102: 996–1003.
Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV : An X chromosome gene regulates hematopoietic stem cell kinetics. Proc Natl Acad Sci USA 1998; 95: 3862–3866.
McGue M, Christensen K : Genetic and environmental contributions to depression symptomatology: evidence from Danish twins 75 years of age and older. J Abnorm Psychol 1997; 106: 439–448.
Christensen K, Gaist D, Jeune B, Vaupel JW : A tooth per child? Lancet 1998; 352: 204.
Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K : The Danish Twin Registry: 127 birth cohorts of twins. Twin Res 2002; 5: 352–357.
Schousboe K, Visscher PM, Henriksen JE, Hopper JL, Sørensen TI, Kyvik KO : Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 2003; 46: 1276–1283.
Nybo H, Gaist D, Jeune B et al: The Danish 1905 cohort: a genetic-epidemiological nationwide survey. J Aging Health 2001; 13: 32–46.
Kristiansen M, Helland A, Kristensen GB et al: X chromosome inactivation in cervical cancer patients. Cancer Genet Cytogenet 2003; 146: 73–76.
Ørstavik KH, Berg K : Skewed X chromosome inactivation pattern in healthy females 83 years or older. Eur J Hum Genet 1998; 6: Suppl 1 A116.
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW : Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239.
Diggle PJ, Liang KY, Zeger SL : Fitting smooth curves to longitudinal data; in: Atkinson AC, Copas JB, Pierce DA, Schervish MJ, Titterington DM (eds): Analysis of Longitudinal Data. Oxford University Press: Oxford, 1994, pp 41–47.
Nance WE : Do twin Lyons have larger spots? Am J Hum Genet 1990; 46: 646–648.
Hall JG : Twins and twinning. Am J Med Genet 1996; 61: 202–204.
Monteiro J, Derom C, Vlietinck R, Kohn N, Lesser M, Gregersen PK : Commitment to X inactivation precedes the twinning event in monochorionic MZ twins. Am J Hum Genet 1998; 63: 339–346.
Neale MC, Cardon LR : Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1992.
Akaike H : Factor analysis and AIC. Psychometrika 1987; 52: 317–332.
Neale MC, Boker SM, Xie G, Maes HH : Mx: Statistical Modeling, 6th ed. Richmond, VA: Department of Psychiatry, MCV, 2002.
Looijenga LH, Gillis AJ, Verkerk AJ, van Putten WL, Oosterhuis JW : Heterogeneous X inactivation in trophoblastic cells of human full-term female placentas. Am J Hum Genet 1999; 64: 1445–1452.
Uehara S, Tamura M, Nata M et al: X-chromosome inactivation in the human trophoblast of early pregnancy. J Hum Genet 2000; 45: 119–126.
Fialkow PJ : Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet 1973; 37: 39–48.
Puck JM, Stewart CC, Nussbaum RL : Maximum-likelihood analysis of human T-cell X chromosome inactivation patterns: normal women versus carriers of X-linked severe combined immunodeficiency. Am J Hum Genet 1992; 50: 742–748.
Machin G, Keith L : Biology of Twins and Other Multiple Pregnancies. New York, Parthenon, 1999.
Goodship J, Carter J, Burn J : X-inactivation patterns in monozygotic and dizygotic female twins. Am J Med Genet 1996; 61: 205–208.
Cattanach BM : Control of chromosome inactivation. Annu Rev Genet 1975; 9: 1–18.
Johnston PG, Cattanach BM : Controlling elements in the mouse. IV. Evidence of non-random X-inactivation. Genet Res 1981; 37: 151–160.
Plenge RM, Percec I, Nadeau JH, Willard HF : Expression-based assay of an X-linked gene to examine effects of the X-controlling element (Xce) locus. Mamm Genome 2000; 11: 405–408.
Albertini RJ, DeMars R : Mosaicism of peripheral blood lymphocyte populations in females heterozygous for the Lesch-Nyhan mutation. Biochem Genet 1974; 11: 397–411.
Tomkins DJ, McDonald HL, Farrell SA, Brown CJ : Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet 2002; 10: 44–51.
Hoffman EP, Pegoraro E : Skewed X inactivation can be inherited as a Mendelian trait in humans. Am J Hum Genet 1995; Suppl 57: A49.
Ørstavik KH, Ørstavik RE, Schwartz M : Skewed X chromosome inactivation in a female with haemophilia B and in her non-carrier daughter: a genetic influence on X chromosome inactivation? J Med Genet 1999; 36: 865–866.
Derom R, Derom C, Vlietinck R : Placentation; in: Keith LG, Papiernik E, Keith DM, Luke B (eds): Multiple Pregnancy: Epitemiology, Gestation and Perinatal Outcome. Parthenon Publishing: New York, 1995, pp 113–128.
Chitnis S, Derom C, Vlietinck R, Derom R, Monteiro J, Gregersen PK : X chromosome-inactivation patterns confirm the late timing of monoamniotic–MZ twinning. Am J Hum Genet 1999; 65: 570–571.
Trejo V, Derom C, Vlietinck R et al: X chromosome inactivation patterns correlate with fetal-placental anatomy in monozygotic twin pairs: implications for immune relatedness and concordance for autoimmunity. Mol Med 1994; 1: 62–70.
Sharp A, Robinson D, Jacobs P : Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet 2000; 107: 343–349.
Hatakeyama C, Anderson C, Beever C, Penaherrera M, Brown C, Robinson W : The dynamics of X-inactivation skewing as women age. Clin Genet 2004; 66: 327–332.
Vickers MA, McLeod E, Spector TD, Wilson IJ : Assessment of mechanism of acquired skewed X inactivation by analysis of twins. Blood 2001; 97: 1274–1281.
Cazzola M, May A, Bergamaschi G, Cerani P, Rosti V, Bishop DF : Familial-skewed X-chromosome inactivation as a predisposing factor for late-onset X-linked sideroblastic anemia in carrier females. Blood 2000; 96: 4363–4365.
Au WY, Ma ES, Lam VM, Chan JL, Pang A, Kwong YL : Glucose 6-phosphate dehydrogenase (G6PD) deficiency in elderly Chinese women heterozygous for G6PD variants. Am J Med Genet 2004; 129A: 208–211.
Acknowledgements
This work was supported by the Research Council of Norway, The Norwegian Cancer Association, US National Institute on Aging (AG-08761), the Danish Interdisciplinary Research Council, and the Danish National Research Foundation to KC, and the Canadian Institutes of Health Research (CIHR) operating grant to AKN. MK and GPSK are Research Council of Norway scholarship recipients. AKN is a CIHR New Investigator. We acknowledge Thore Egeland for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristiansen, M., Knudsen, G., Bathum, L. et al. Twin study of genetic and aging effects on X chromosome inactivation. Eur J Hum Genet 13, 599–606 (2005). https://doi.org/10.1038/sj.ejhg.5201398
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201398