Abstract
Spinal muscular atrophy (SMA) is caused by insufficient levels of survival motor neuron (SMN) protein. Recently, we found that sodium 4-phenylbutyrate (PB), a well-tolerated FDA approved drug, enhances SMN gene expression in vitro. We provide here the first evidence that oral administration of PB (triButyrate®) significantly increases SMN expression in leukocytes of SMA patients. This finding provides a strong rationale to further investigate the effects of PB as also supported by preliminary clinical data.
Similar content being viewed by others
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disorder characterized by degeneration of anterior horn cells of the spinal cord. SMA is caused by loss of the functional survival motor neuron gene (SMN1).1 However, all patients have one or more copies of the SMN2 gene, nearly identical to SMN1.2,3 Both genes encode the SMN protein but, due to alternative splicing, the level of functional protein produced by SMN2 is insufficient to protect from disease. The phenotype can range from very severe to mild (type I to III) and the clinical severity inversely correlates with the amount of functional SMN protein.4 No cure for SMA is available at present. Increasing SMN2 gene expression could be of invaluable therapeutic importance. Recently, we have shown that treatment of fibroblast cultures from SMA patients with sodium 4-phenylbutyrate (PB), a chromatin hyperacetylating agent, leads to a significant increase in SMN2 gene expression.5 Moreover, in a preliminary clinical study, we observed an improvement of motor function in SMA children following treatment with PB (triButyrate®).6 We now performed further molecular studies which provide evidence that PB is effective in enhancing SMN expression in peripheral blood leukocytes.
Subjects and methods
Six SMA patients (P1–P6) and three parents (M2, M3, F6) were enrolled for the present pilot trial. Four patients had SMA type II (P1–P4). P1 is a 2.5-year-old boy who had lost the ability to sit unaided and to control the upright position of the head. Patients P2 (5 years), P3 and P4 (9 years) are all able to sit independently. Two patients had SMA type III (P5, P6). P5 is a 38-year-old male who is now only able to walk with aid and P6 is a 15-year-old female who still is able to walk independently. All patients had three copies of the SMN2 gene.5 The trial was approved by the Ethical Committee of the Catholic University. A written informed consent was obtained from all patients/parents. TriButyrate® was orally administered at 500 mg/kg/d (maximum dose 19 g/d), divided into six doses (every 4 h) for 7 days. Blood samples were taken from patients and parents during the morning of day 0 (T0, baseline) and days 1–4 (T1–T4) and 7 (T7) of treatment, usually 1–3.5 h after drug administration, and from five healthy untreated controls on five consecutive days (T0–T4). Total RNA was extracted by Trizol from leukocytes immediately after hypotonic lysis of samples and all blood samples were processed equally.
Real-time PCR
SMN full-length (SMN-fl) transcripts and transcripts lacking exon 7 (Δ7) were measured by real-time RT-PCR using ABI-PRISM 7700 Sequence Detector System (Applied Biosystems) as described elsewhere.5 Transcripts were amplified at least twice in triplicate or quadruplicate. SMN transcript levels were calculated by comparing SMN versus glyceraldehyde-3-phosphate dehydrogenase transcripts, whose expression is apparently not affected by PB.5 The relative amounts of SMN-fl and Δ7 transcripts in samples obtained from treated patients/parents and untreated controls were normalized versus those of T0.
Myometry
Muscle strength was assessed using a hand-held dynamometer (Citec, CIT Technics BV).7 Patients were tested independently four times by two raters, and the highest measure of the maximal voluntary isometric contraction was selected. Inter-rater reliability was assessed by calculating the intraclass correlation coefficient (ICC)8 using SPSS 10.0 for Windows.
Statistical analysis
Significance of real-time PCR data was assessed by ANOVA and by testing the null hypothesis of no effect of treatment with Student's t for independent variables and Fisher's tests (Winstat 4.01 and SPSS 10.0.1 for Windows software). For myometry data, χ2 test and nonparamentric Friedman test for multiple samples were used. P-values <0.05 were accepted as significant.
Results
SMN expression studies
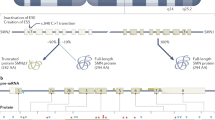
SMN mRNA analysis showed for all patients a marked increase in relative SMN-fl transcript levels in one or more blood samples obtained during PB administration compared to baseline (Table 1, Figure 1a). The mean increase in patients transcript levels ranged from 0.4-fold for P6 up to 2.4-fold observed in P4, and from 0.9- to 1.7-fold in the parents. The relative amount of SMN-fl mRNA during treatment varied considerably, both among the different subjects and the different blood samples of the same subject. Despite the day-to-day variations, data were found statistically significant (t-test and F-ratio values >1, P≤0.03), thus the null hypothesis of no effect of PB was rejected. To investigate whether SMN-fl transcript levels are subject to physiologic variation, SMN expression was studied in five healthy untreated controls during 5 days. A slight-to-moderate variation in SMN-fl levels of T1–T4 versus T0 was observed in the controls with mean variation between 4.2 and 16.5% (Figure 1b). Variation in SMN-fl levels in treated patients/parents versus controls was statistically significant.
SMN expression studies in PB-treated and-untreated subjects. (a) Variation of SMN -fl transcript levels in leukocytes of patients and parents at the single days (T1–T4, T7) of PB treatment relative to baseline levels. Mean values and SD are indicated by long and short horizontal bars, respectively. M2, M3, F6: mothers of P2, P3 and father of P6, respectively. (b) Variation of SMN mRNA in healthy untreated controls during 4 days relative to mean level at T0. (c) SMN-fl (◊) and Δ7 (Δ) transcript studies. (d) Percent of SMN-fl transcript levels in patients before treatment relative to that of unaffected individuals. RIS (Reference Internal Standard) indicates the average amount of SMN-fl mRNA in five unaffected individuals at T0. Error bars indicate SD.
To investigate whether PB influences inclusion/exclusion of exon 7, we studied SMN-fl and Δ7 transcripts in some blood samples. We found a slight reduction of Δ7 transcripts in all blood samples analyzed suggesting an effect of PB on both promoter activation and alternative splicing (Figure 1c).
To estimate the percentage of the SMN-fl transcript levels in our patients before treatment respective to that of unaffected subjects, their baseline SMN mRNA levels were compared to a reference internal standard (RIS), calculated as average amount of SMN-fl mRNA of the controls at T0. The relative SMN-fl transcript levels were 22–28 and 47–48% of control levels in the type II and type III patients, respectively (Figure 1c).
Clinical observations
All subjects tolerated the drug well except for P6 who on day 2 complained of dizziness and tinnitus, which resolved immediately after reducing dosage from 18 to 12 g/d. Full blood counts and liver function tests, performed for all subjects at days 0 and 7, did not show relevant changes.
The first patient studied (P5) reported a reduction of hand tremor at day 3 of treatment and subsequently complete absence of tremors lasting for 4 days after the end of the trial. The second patient (P1), a young child with severe type II phenotype, showed a slight improvement in head and trunk control. These subjective improvements prompted us to perform myometry in the other four patients on days 0 and 7 (Table 2). Muscle strength was found significantly increased in the group of patients (P=0.041). When analyzed separately, improvement of muscle strength was most evident for P4, less pronounced in P2 and P3, whereas no changes in muscle strength were found for P6.
Discussion
We report here that SMN2 gene expression can be increased in leukocytes of SMA patients by oral administration of PB. The small amounts of blood samples obtained from the patients did not allow to perform protein studies. PB is an FDA approved drug which has been used for several years for the treatment of young patients with urea cycle disorders and is well tolerated.9 Recently, evidence was given that PB can cross the blood–brain barrier.10
A drawback to the use of PB is its short-half life (0.8–1 h). Previous pharmacokinetic studies have shown rapid changes in serum levels of PB in treated subjects with sickle cell disease.11 The variability in SMN gene expression following PB administration observed in our patients may be related to the varying levels of plasmatic PB concentrations at different intervals between drug administration and blood sampling.
When we investigated on the extent of reduction in SMN-fl levels in our patients before treatment compared to unaffected individuals, we found that the type II and III patients had approximately 25 and 50%, respectively, of SMN-fl levels of controls. If we consider that in all patients a more than 100% increase in SMN-fl transcripts was detected in at least one blood sample during treatment we may speculate that SMN-fl levels in leukocytes of SMA type II patients could transiently exceed the baseline levels of type III patients and that the latter could achieve levels similar to that of controls.
The observation of an increase in muscle strength after 1 week of treatment was unexpected and may be the result of a placebo effect. However, a similar trend was found also in our recent open pilot study.10 Regarding our present data, it may be not by chance that the patient (P4) with the highest mean increase in SMN transcript levels showed also the more obvious improvement in muscle strength and the case with the lowest mean increase (P6 who has taken a 33% reduced dosage) had no change in muscle strength. Further data on the effect of PB on muscle strength will be provided by the ongoing placebo-controlled double-blind clinical trial.
Our observation that PB increases SMN expression was made on peripheral blood leukocytes. Although we do not have direct information on the effects on muscle or motor neuron cells, our preliminary clinical data suggest that PB improves motor function in SMA patients.6
In conclusion, while an effect of PB on SMN protein expression has still to be determined, we provide here the first evidence that SMN transcript levels can be enhanced in SMA patients by drug administration.
References
Lefebvre S, Bürglen L, Reboullet S et al: Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995; 80: 155–165.
Vitali T, Sossi V, Tiziano F et al: Detection of the survival motor neuron (SMN) genes by FISH: further evidence for a role of SMN2 in the modulation of disease severity in SMA patients. Hum Mol Genet 1999; 8: 2525–2532.
Feldkötter M, Schwarzer V, Wirth R et al: Quantitative analysis of SMN1 and SMN2 based on real-time LightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet 2002; 70: 358–368.
Lefebvre S, Burlet P, Liu Q et al: Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 1997; 16: 265–269.
Andreassi C, Angelozzi C, Tiziano FD et al: Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. Eur J Hum Genet 2004; 12: 59–65.
Mercuri E, Bertini E, Messina S et al: Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromusc Disord 2004; 14: 130–135.
Merlini L, Mazzone ES, Solari A, Morandi L : Reliability of hand-hold dynamometry in spinal muscular atrophy. Muscle Nerve 2002; 26: 64–70.
Shrout PE, Fleiss JL : Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86: 420–428.
Maestri NE, Brusilow SW, Clissold DB, Bassett SS : Long term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med 1996; 335: 855–859.
Berg S, Serabe B, Aleksic A et al: Pharmacokinetics and cerebrospinal fluid penetration of phenylacetate and phenylbutyrate in the nonhuman primate. Cancer Chemother Pharmacol 2001; 47: 385–390.
Dover GJ, Brusilow S, Charache S : Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood 1994; 84: 339–343.
Acknowledgements
We thank the patients and families for their kind cooperation and are grateful to Valentina Leo for her help in taking the blood samples. We also thank Fyrklövern Scandinavia AB, Sweden, for providing the drug triButyrate®. This study was supported by the Associations Families of SMA USA, Families of SMA Italy, ASAMSI, and by Telethon (Grant GGP030109).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brahe, C., Vitali, T., Tiziano, F. et al. Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur J Hum Genet 13, 256–259 (2005). https://doi.org/10.1038/sj.ejhg.5201320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201320
Keywords
This article is cited by
-
Evaluation of the orally bioavailable 4-phenylbutyrate-tethered trichostatin A analogue AR42 in models of spinal muscular atrophy
Scientific Reports (2023)
-
Epigenetics in Alzheimer’s Disease: Perspective of DNA Methylation
Molecular Neurobiology (2018)
-
Applicability of Histone Deacetylase Inhibition for the Treatment of Spinal Muscular Atrophy
Neurotherapeutics (2013)
-
Clinical and molecular cross-sectional study of a cohort of adult type III spinal muscular atrophy patients: clues from a biomarker study
European Journal of Human Genetics (2013)
-
Spinal muscular atrophy
Orphanet Journal of Rare Diseases (2011)