Key Points
-
This manuscript discusses an investigation of the relationship between chemical parameters of popular soft drinks and enamel erosion.
-
The effects of toothbrushing after exposure to soft drinks are described as a function of the chemical parameters of the drink.
-
Clinically relevant times for erosion and brushing are used in this in vitro study.
-
A correlation is drawn between the amount of tissue loss caused by erosion, and the extent of the softened layer, in that drinks which cause greater erosion also cause a thicker softened layer.
Abstract
Objective To investigate how enamel loss due to erosion, and due to cycling of erosion and abrasion, depends on compositional parameters of soft drinks, and particularly whether the thickness of the erosive softened layer is a function of drink composition.
Setting University dental hospital research laboratory in the UK, 2004.
Materials and methods Six drinks were chosen based on their popularity and composition: apple juice, orange juice, apple drink, orange drink, cranberry drink and 'ToothKind' blackcurrant drink. Group A samples (n = 36) were exposed to soft drinks at 36°C for six consecutive 10 minute periods. Group B samples (n = 36) were subjected to alternating erosion and toothbrushing, repeated six times. Enamel loss was measured using optical profilometry.
Results Group A: significant enamel loss was seen for all drinks (p < 0.001). Erosion was correlated with pH and calcium concentration but not phosphate concentration or titratable acidity. Group B: significant additional material loss due to toothbrush abrasion occurred with all drinks. Abrasive enamel loss differed between the drinks and was positively correlated with drink erosive potential.
Conclusion Enamel loss by erosion is exacerbated by subsequent abrasion. The amount of softened enamel removed by toothbrushing is a function of the chemical composition of the erosive medium.
Similar content being viewed by others
Introduction
Dental erosion may be defined as tooth wear due to dissolution of the dental hard tissues by acids without the involvement of bacteria.1 Erosion is becoming more commonly recognised in both adults and children, with over half of 15-18-year-olds exhibiting incisal erosion.2 The severity of erosion is related to several factors, including the chemical properties of the erosive medium, and the frequency and method of contact between acid and tooth. It is also related to the effectiveness of the protective mechanisms in the oral cavity, including salivary composition, flow rate, and buffering capacity, pellicle formation, clearance rates, and individual dental anatomy.3
Acids which cause dental erosion may be extrinsic or intrinsic in origin. Dietary acids are the most extensively studied aetiological agent, and can be said to be the most important extrinsic factor.4 In particular, dental erosion by acidic soft drinks appears to be a growing problem and has been the subject of numerous studies in vitro and in situ.4,5,6 The erosive potential of a soft drink depends on chemical parameters, including pH, titratable acidity, and calcium and phosphate concentrations.7,8 The titratable acidity is a measure of the total acid content of a drink, and is thought by some authors to be a more accurate means of predicting erosive potential than pH.9,10,11 Exposure to an erosive medium results in irreversible loss of enamel from the tooth surface, and a layer of softened enamel 0.5-5 μm thick.12,13
Dental abrasion is defined as the wearing away of the dental hard tissues through physical means other than teeth. Brushing the teeth immediately after consuming acidic products accelerates enamel loss, as the softened layer is susceptible to abrasion.14,15 Enamel softening is reversible, whereby salivary calcium and phosphate lead to the remineralisation of the softened tissue.12 If the time for remineralisation of enamel is not sufficient and abrasion occurs immediately after erosion, the softened tooth enamel is also removed.14,15
Little research has taken place using realistic, clinically relevant acid exposure and brushing times. Typical acid exposure times are in the region of several hours or days8,16,17 and brushing studies often use several hundred or thousand brushing strokes.18,19,20 In addition, many previous in vitro studies are not readily comparable since information on exposure and stirring technique were not reported and therefore rate of liquid flow or linear velocity is unknown. Variables such as these have important relevance to erosion, thus these studies may not accurately depict the impact of frequent soft drink consumption on enamel.
The aim of this in vitro study was twofold. Firstly, the erosive enamel loss due to soft drinks, and erosive-abrasive enamel loss due to cycling of soft drinks and toothbrush abrasion, was investigated under carefully controlled conditions. Secondly, the dependence of the thickness of the softened layer, and the amount of enamel removed by abrasion, was investigated as a function of the composition and erosive potential of the soft drink. Optical profilometry was used to quantify enamel loss since it is a sensitive, non-destructive technique.
Method
Soft drinks
pH was measured using a pH meter (HI 9321 Microprocessor pH meter; Hanna Instruments, Leighton Buzzard, UK). Titratable acidity of 100 ml soft drink was measured by titrating to pH 7.00 using 1 mol l−1 NaOH. Calcium concentration was measured using atomic absorption spectroscopy and phosphate concentration was measured using a modification of the Chen method.21 The measurements were performed in triplicate.
Six soft drinks were selected on the basis of sales figures and variation in titratable acidity and pH. This selection is discussed further in the Discussion section.
-
Tesco Pure Apple Juice™ (Tesco Stores, Cheshunt, UK), high pH/low titratable acidity
-
Tropicana Pure Premium Orange Juice™ (Tropicana UK Ltd, Leicester, UK), high pH/high titratable acidity
-
Ocean Spray Cranberry Classic Juice Drink™ (Ocean Spray International, Gerber Foods Soft Drinks, Bridgwater, Somerset, UK), low pH/low titratable acidity
-
Robinsons Apple 'Fruitshoot' Drink™ (Robinsons Soft Drinks, Chelmsford, UK), low pH/high titratable acidity
-
Capri Sun Orange Drink™ (Coca Cola Enterprises, Uxbridge, UK), mid-range pH/mid-range titratable acidity
-
Ribena 'ToothKind' Blackcurrant Drink™ (GlaxoSmithKline Consumer Healthcare, Brentford, UK), high pH/low titratable acidity
Sample preparation
Seventy-two enamel samples were cut from the sides of sound, permanent human molars extracted from patients living in an area with unfluoridated water, using a diamond-edged saw (Microslice 2, Metal Research, Cambridge, UK). Samples were placed in polyurethane moulds measuring 8 × 5 × 2 mm and embedded in epoxy resin (Hitek Electronic Materials, Scunthorpe, UK) for 24 h. Samples were ground and polished using a lapping and polishing unit (Kemet International, Maidstone, UK) using silicon carbide discs of up to 1200 grit to expose a smooth, flat area of enamel. In addition to exposing a flat, smooth area for investigation, this process also removed the surface enamel which may be chemically altered by processes occurring in the oral environment, and exposed underlying enamel which is more chemically homogeneous. A baseline measurement of each sample was taken using a non-contact optical profilometer (Proscan 2000, Scantron, Taunton, UK), as described below, to ensure that it was sufficiently flat and smooth. The exposed enamel area was then partly covered with white PVC tape, allowing a 2 mm wide window to be exposed to the drinks. The samples were randomly allocated to six groups of six samples for each of groups A and B.
Group A: erosion
Six enamel samples were simultaneously exposed to 500 ml of fresh drink at 36°C. The samples were attached at equal intervals around the circumference of a plastic Petri dish 8.5 cm in diameter. The disc was rotated using a Cat R50D overhead stirrer (Bennett Scientific Ltd, Newton Abbot, UK) at 110 rpm, corresponding to a linear sample velocity of 0.5 m s−1. The stirrer was positioned such that the disc was ∼ 2 cm from the side of the beaker and ∼ 4 cm from the base of a 1 l beaker. After 10 min, the samples were removed and rinsed with distilled water. The adhesive tape was removed and the samples were allowed to dry in air for 30 min, and then profiled to measure enamel loss. Each sample was then taped again in the same position. This was repeated for a further five exposures for each of the six drinks.
Group B: erosion and abrasion
Group B samples were exposed to soft drinks in the same way as group A samples. After each erosive challenge the samples were then subjected to an abrasive challenge to represent toothbrush abrasion. A slurry of a non-fluoridated, calcium carbonate-based, British Standard Institute toothpaste (GlaxoSmithKline, Weybridge, Surrey, UK) was prepared in a 1:3 ratio with tap water. Two toothbrush heads, (Oral-B, size 35, regular, Gillette, London, UK) were inserted into a holder loaded with a 200 g weight, horizontally positioned above a tray filled with toothpaste slurry, as described previously.22 Samples were brushed with 25 strokes of the toothbrush. After brushing, the samples were rinsed with distilled water and allowed to dry in air for 30 min. Samples were then profiled and then subjected to a further five erosion–profile–abrasion–profile cycles.
Measurement of erosion
Erosion was measuring using a non-contact profilometer (Proscan, Scantron, Taunton, UK). For each sample, the depth of enamel loss was measured at five equally spaced positions and a mean value taken. Erosion was measured after each erosive challenge for group A samples, and after each erosive challenge and each abrasive challenge for group B samples.
Statistical analysis
A one-way ANOVA of erosion data after 60 min total erosion time, followed by a multiple range test (Bonferroni) at a 95% confidence level, was performed to identify statistically homogeneous groups in each of group A and group B data. Paired t-tests were performed to identify whether or not there were statistically significant differences between erosive and erosive-abrasive material loss after 60 min total erosion time for each drink. The correlation between erosive and abrasive material loss was investigated by calculating the non-parametric correlation coefficient (Spearman's rho) between the mean material loss due to erosion per cycle and the mean material loss due to abrasion per cycle, for each drink (group B specimens).
Results
The chemical properties of the drinks are shown in Table 1. It can be seen that the apple drink had the lowest pH (2.74), whilst the orange juice had the highest (3.95). The other drinks had pH ranging between these drinks. Titratable acidity ranged from 2.28 × 102 mmol l−1 ('ToothKind' blackcurrant drink) to 10.6 × 102 mmol l−1 (orange juice). Calcium concentration ranged from 0.40 mmol l−1 (apple drink) to 5.81 mmol l−1 ('ToothKind' blackcurrant drink). Phosphate concentration ranged from 0.11 mmol l−1 (cranberry drink) to 1.20 mmol l−1 (apple juice).
Group A: erosion
There was a statistically significant difference between the erosion caused by the drinks (p < 0.001, Fig. 1). There was a very large initial enamel loss with cranberry drink (15.5 μm (sd = 3.1 μm)). For the other drinks, erosion during the first exposure was not substantially greater than erosion over any of the subsequent exposures. In order of least to most erosive, the drinks could be classified into homogeneous groups as follows: 'ToothKind' blackcurrant drink,a orange juice,a,b orange drink,b,c apple juice,capple drink,d cranberry drink,d where superscript letters indicate statistically homogeneous groups.
Group B: erosion and abrasion
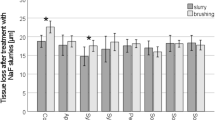
There was a statistically significant difference between the erosive/abrasive loss caused by the drinks (p < 0.001, Fig. 2). The enamel loss due to different drinks followed a similar pattern to group A and could be classified into homogeneous groups as follows: 'ToothKind' blackcurrant drink,a orange juice,a,b apple juice,b orange drink,b,c cranberry drink,c,d apple drink,d where superscript letters indicate statistically homogeneous groups.
Toothbrush abrasion caused additional enamel loss after a total of 60 min erosive challenge. This loss was statistically significant for all drinks: 'ToothKind' blackcurrant drink (p < 0.001), orange juice (p < 0.001), apple juice (p = 0.003), orange drink (p = 0.003), cranberry drink (p = 0.013) and apple drink (p = 0.001). The mean amount of abrasive enamel loss after exposure to the different drinks was: 'ToothKind' blackcurrant drink 0.57 μm, orange juice 0.86 μm, apple juice 0.73 μm, orange drink 1.20 μm, cranberry drink 1.25 μm, apple drink 2.16 μm.
There was a positive correlation between erosive and abrasive enamel loss (ρ = 0.771), which was significant at a 95% confidence level.
Discussion
The drinks selected for this study displayed a wide range of pH and titratable acidities. From our own and other investigations,23,24 we identified a typical range for fruit drinks of 2.5 < pH < 4 and a titratable acidity of 20–150 mmol l−1 OH− required to raise the pH to 7. From a number of fruit drinks and juices with high sales figures in the UK (provided by AC Nielsen, Oxford, UK), we selected six drinks to represent the extremes (high/low pH, high/low titratable acidity) and the averages (mid-range pH and titratable acidity) of the range found. The stirring speed of 0.5 m s−1 was selected based on mean drinking rates such that it is typical of clinical drink flow rates and is not affected by very small changes in velocity, such as occurs at lower velocities.25
Since all of the drinks tested had a pH of below 4, it was to be expected that exposure to each of them resulted in a progressive loss of enamel. The degree of erosion can be correlated with some of the chemical parameters. The two most erosive drinks have the lowest pH values, the two intermediate drinks have intermediate pH values, and the two least erosive drinks have the highest pH values. There is no clear correlation with titratable acidity; in fact one of the most erosive drinks (cranberry drink) and the least erosive drink ('ToothKind' blackcurrant drink) have very similar titratable acidities.
This suggests that pH is more important than titratable acidity in determining erosive potential. This contradicts other studies which indicate that erosive potential is influenced by titratable acidity.7,8,10 This can be explained with reference to experimental conditions. This study employed a large volume of solution, thorough agitation, and clinically relevant exposure times, producing a constant composition chemical environment. Other studies have used smaller solution volumes and longer exposure times, resulting in a change in the composition of the drink over the duration of the experiment. Under changing conditions the buffering capacity (titratable acidity) of the drink can be expected to be a significant factor, however under constant composition the pH would be likely to be the more important parameter. The conditions described in the present study can be considered to be the more clinically relevant, since in vivo drinks are usually not held in contact with the teeth for prolonged periods, but are rather continually refreshed during drinking.
There was a negative correlation between calcium concentration and erosion, but no clear relationship between phosphate concentration and erosion. The importance of calcium can be explained with reference to thermodynamics, since calcium ions in solution inhibit further detachment of calcium ions from the enamel surface. The same argument might be thought to be applicable to phosphorus concentration too; however the fact that phosphorus has little or no correlation with erosion might be explained by the observation that calcium ions are detached from the hydroxyapatite surface before phosphate ions.26 In addition, given the low pH values, most of the inorganic phosphorus in the drinks is in the form of H2PO4− and very little in the form of PO43−. Other studies have shown that calcium is substantially more effective than phosphate at reducing enamel erosion in modified drinks.27
The amount of enamel removed by toothbrush/toothpaste was different for samples exposed to different drinks, although the brushing method was the same for all samples. This implies that the thickness of the softened layer is dependent on the chemical composition of the erosive drink. This has not been reported before. Our results indicate that drinks which cause the greatest erosion also cause the greatest subsurface softening. This would not necessarily have been expected, since it is logical that a more aggressive solution would remove surface enamel more quickly, but not necessarily that it would also penetrate more rapidly into subsurface enamel. It can be concluded, however, that this is the case, and that drinks which rapidly dissolve surface enamel also diffuse further into the enamel bulk in a given timeframe.
The toothpaste used in this study did not contain fluoride, as the aim of the study was to compare mineral loss due to chemical dissolution and mechanical abrasion, without the additional complication of chemical erosion protection which may be offered by fluoride. Although the potential of fluoride to reduce caries has been well documented, the effect on erosion is less clear, particularly in combination with abrasion, which is the most common delivery mechanism for intra-oral fluoride. Further research is necessary to investigate the interaction of chemical dissolution, mechanical abrasion and chemical inhibition of dissolution by fluoride.
Erosion in vivo is considerably less rapid than in vitro owing to the protective effect of the saliva and acquired pellicle. In addition, polished enamel such as used in this study is eroded more rapidly than natural enamel surfaces.28 As such, the rate of material loss observed in this study can be considered to be much more rapid that that which would occur in vivo. Furthermore, the BSI toothpaste used in this study had a Relative Dentine Abrasion index of ∼ 100, which is representative of the more abrasive toothpastes currently available on the market. This study therefore represents an exaggeration of the erosion which may occur in vivo. The correlation between erosive material loss and abrasive material loss, and therefore between erosive potential of the drink and depth of the softened layer, is thought to be of potential clinical significance. Another conclusion which can be drawn from our results is that studies which investigate only erosion may underestimate the potential harm caused by more erosive drinks, since these do not take into account the differing degrees of softening caused by the drinks.
The results of this in vitro study demonstrate that enamel softened by erosion is readily susceptible to abrasion through toothbrushing. In addition, drinks which cause the greatest erosion also result in a greater softened zone at the enamel surface. Under constant composition conditions, thought to be relevant to those found in vivo, pH and calcium are more important than titratable acidity or phosphate in determining erosive and softening potential of drinks. Toothbrushing following the consumption of acidic beverages should be postponed to minimise or avoid enamel loss.
References
Imfeld T . Dental erosion. Definition, classification and links. Eur J Oral Sci 1996; 104: 151–155.
Nunn J H, Gordon P H, Morris A J, Pine C M, Walker A . Dental erosion - changing prevalence? A review of British national children's surveys. Int J Paediatr Dent 2003; 13: 98–105.
Meurman J H, ten Cate J M . Pathogenesis and modifying factors of dental erosion. Eur J Oral Sci 1996; 104: 199–206.
Zero D, Lussi A . Etiology of enamel erosion: intrinsic and extrinsic factors. In Addy M, Embery G, Edgar W M, Orchardson R (eds) Tooth Wear and Sensitivity. Clinical Advances in Restorative Dentistry. pp 122–139. London: Taylor and Francis, 2000.
Dugmore C R, Rock W P . A multifactorial analysis of factors associated with dental erosion. Br Dent J 2004; 196: 283–286.
Al-Dlaigan Y H, Shaw L, Smith A J . Dental erosion in a group of British 14-year-old school children. Part II: Influence of dietary intake. Br Dent J 2001; 190: 258–261.
Lussi A, Jaggi T, Scharer S . The influence of different factors on in vitro enamel erosion. Caries Res 1993; 27: 387–393.
Larsen M J, Nyvad B . Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res 1999; 33: 81–87.
Shaw L, Smith A J . Dental erosion - the problem and some practical solutions. Br Dent J 1998; 186: 115–118.
Zero D T . Etiology of dental erosion - extrinsic factors. Eur J Oral Sci 1996; 104: 162–177.
Grobler S R, Jenkins G N, Kotze D . The effects of the composition and method of drinking of soft drinks on plaque pH. Br Dent J 1985; 158: 293–296.
Attin T, Buchalla W, Gollner M, Hellwig E . Use of variable remineralization periods to improve the abrasion resistance of previously eroded enamel. Caries Res 2000; 34: 48–52.
Eisenburger M, Addy M . Influence of liquid temperature and flow rate on enamel erosion and surface softening. J Oral Rehab 2003; 30: 1076–1080.
Davis W B, Winter P J . The effect of abrasion on enamel and dentine after exposure to dietary acid. Br Dent J 1980; 148: 253–256.
Van der Weijden F, Danser M M . Toothbrushes: benefits versus effects on hard and soft tissues. In Addy M, Embery G, Edgar W M, Orchardson R (eds) Tooth Wear and Sensitivity. Clinical Advances in Restorative Dentistry. pp 217–236. London: Taylor and Francis, 2000.
Amaechi B T, Higham S M, Edgar W M . Use of transverse microradiography to quantify mineral loss by erosion in bovine enamel. Caries Res 1998; 32: 351–356.
Eisenburger M, Addy M . Evaluation of pH and erosion time on demineralisation. Clin Oral Investigations 2001; 5: 108–111.
Bartlett D W, Smith B G, Wilson R F . Comparison of the effect of fluoride and non-fluoride toothpaste on tooth wear in vitro and the influence of enamel fluoride concentration and hardness of enamel. Br Dent J 1994; 176: 346–348.
Torrado A, Valiente M, Munoz C A . Cleaning power and abrasivity of a new toothpaste based on ion-exchange resins. Am J Dent 2004; 17: 80–84.
Litonjua L A, Andreana S, Bush P J, Tobias T S, Cohen R E . Wedged cervical lesions produced by toothbrushing. Am J Dent 2004; 17: 237–240.
Chen P S, Toribara T Y, Warner H . Microdetermination of phosphorus. Anal Chem 1956; 28: 1756–1758.
Dyer D, Addy M, Newcombe R G . Studies in vitro of abrasion by different manual toothbrush heads and a standard toothpaste. J Clin Periodontol 2000; 27: 99–103.
Feldman M, Barnett C . Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gastroenterology 1995; 108: 125–131.
Lussi A, Jaeggi T, Zero D T . The role of diet in the aetiology of dental erosion. Caries Res 2004; 38: 34–44.
Shellis R P, Finke M, Eisenburger M, Parker D M, Addy M . Relationship between enamel erosion and liquid flow rate. Eur J Oral Sci 2005; 113: 232–238.
Dorozhkin S V . Surface reactions of apatite dissolution. J Colloid Interface Sci 1997; 191: 489–497.
Barbour M E, Parker D M, Allen G C, Jandt K D . Enamel dissolution in citric acid as a function of calcium and phosphate concentrations and degree of saturation with respect to hydroxyapatite. Eur J Oral Sci 2003; 111: 428–433.
ten Cate J M . Chemistry of demineralization and remineralization of enamel and dentine. In Addy M, Embery G, Edgar W M, Orchardson R (eds) Tooth Wear and Sensitivity. Clinical Advances in Restorative Dentistry. pp 153–160. London: Taylor and Francis, 2000.
Acknowledgements
We gratefully acknowledge GlaxoSmithKline for funding this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Hemingway, C., Parker, D., Addy, M. et al. Erosion of enamel by non-carbonated soft drinks with and without toothbrushing abrasion. Br Dent J 201, 447–450 (2006). https://doi.org/10.1038/sj.bdj.4814073
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4814073
This article is cited by
-
Validation of an erosive tooth wear risk factors questionnaire for adolescents
Clinical Oral Investigations (2022)
-
Oral diseases and oral health related behaviors in adolescents living in Maasai population areas of Tanzania: a cross-sectional study
BMC Pediatrics (2019)
-
Erosive characteristics and fluoride content of cola-type drinks
British Dental Journal (2016)
-
Erosive potential of energy drinks on the dentine surface
BMC Research Notes (2013)
-
Thickness of softened human enamel removed by toothbrush abrasion: an in vitro study
Clinical Oral Investigations (2010)