Key Points
-
This paper suggests that the use of Alpron disinfectant may play a short term role in controlling microbial contamination of DUWLs.
-
The methods described could be readily used in general dental practice.
-
Dental staff and patients should benefit from the reduction in microbial loads in DUWLs.
Abstract
Aims To assess the efficacy of a disinfectant, Alpron, for controlling microbial contamination within dental unit water lines.
Methods The microbiological quality of water emerging from the triple syringe, high speed handpiece, cup filler and surgery hand wash basin from six dental units was assessed for microbiological total viable counts at 22°C and 37°C before and after treatment with Alpron solutions.
Results The study found that the use of Alpron disinfectant solutions could reduce microbial counts in dental unit water lines to similar levels for drinking water. This effect was maintained in all units for up to six weeks following one course of treatment. In four out of six units the low microbial counts were maintained for 13 weeks.
Conclusions Disinfectants may have a short term role to play in controlling microbial contamination of dental unit water lines to drinking water quality. However, in the longer term attention must be paid to redesigning dental units to discourage the build up of microbial biofilms.
Similar content being viewed by others
Main
It was reported as early as 1963 that water from dental unit water lines can be heavily contaminated with micro-organisms1 and confirmed more recently.2,3,4,5,6,7,8,9 One study has demonstrated raised levels of antibodies to Legionella species in dental staff suggesting an occupational exposure.10 However, there is little additional epidemiological evidence that microbial contamination of dental unit water lines constitutes a significant risk of infection to either patients or their dentists. This may be more due to difficulties in collecting the appropriate data. Among immunocompromised individuals there is undoubtedly a potential for infection via this route.11
The source of bacterial contamination within the dental unit water supply is thought to be caused by micro-colonies of proliferating bacteria, fungi and protozoa on the inner surface of the water lines, forming a biofilm.5 A wide range of micro-organisms can be isolated which include environmental organisms, opportunistic pathogens, such as Pseudomonas spp. and human pathogens, such as Legionella pneumophila.9 Whilst many of these organisms may originate from incoming mains water supplies, of concern is the detection of micro-organisms commonly found in the oral cavity,9,12,13 such as Candida spp. and Streptococcus spp. It seems prudent therefore that reasonable efforts should be made to ensure that potable (drinking water) quality water emerges from dental unit handpieces. Current BDA guidelines14 recommend flushing of water lines in between patients although high microbial loads may still persist.6,15,16 The problem of microbial contamination of dental unit waterlines (DUWLs) is compounded by the intricacy and complexity of dental units for which there appear to be no immediate solutions. The long-term solution to the problem lies in redesigning the water supply system within dental units to eliminate stagnant areas and biofilm build up. In the shorter term, disinfectants may have a role to play in controlling the levels of microbial contamination within dental unit water lines to more acceptable levels.
Aim
To assess the efficacy of a biofilm removal agent (citric acid and Sodium-p-toluolsulphonechloramide <0.2%) and disinfectant (EDTA and sodium tosylchloramide) solution marketed under the trade name of Alpron (Quality Water Specialists Ltd, Yorkshire) for the control of microbial contamination of DUWLs.
Methods
Alpron disinfectant
The disinfectant system marketed as Alpron comprises a three component system specifically designed for the removal and control of biofilm formation within the narrow bore plastic water lines of a dental unit. The initial biofilm removal solution consists of a 1–2% sodium hypochlorite solution applied to the DUWLs at an initial temperature of 50°C for a period of 30 minutes. This is followed by a second solution containing alkylamines, complexing agents, tensides and defoamers applied to the DUWS at an initial temperature of 60°C for 30 minutes. The third solution, a 1% solution of sodium-p-toluolsulfonechloramide and sodium ethylenediamine tetra actetic acid (1% Alpron) was added to the reservoir that supplies the water to the dental handpieces and triple syringe.
Dental units
The dental units used in this study were manufactured by A-Dec and were approximately five years old. The water supply to the handpieces and triple syringe was provided by the reservoir containing the 1% Alpron solution. An internal control was provided by the water supply to the cup filler, derived from the hospital mains water supply via the dental unit's internal plastic pipework. An external control was provided from the wash hand basin taps adjacent to each unit, the water supply for which was derived from the main hospital water supply.
Sampling
Samples were collected at baseline and six days after the initial disinfection treatment, followed at weekly intervals for six weeks. Further samples were taken at 10 and 13 weeks. Following collection, each specimen was placed in a coolbag with ice packs whilst being transported to the laboratory. Specimens were returned to the laboratory for processing within one hour of collection.
Sample processing
Equal volumes of sample water were added to 5 ml of inactivating solution (3% Tween 80, 3% Saponin, 0.1% histidin and 0.1% cystein as per manufacturer's instructions). Samples were analysed for total viable counts (TVCs) at 22°C and 37°C using a standard pour plate method.17 Briefly this comprised taking 1 ml of the water sample and dispensing into four empty sterile 90 mm plastic petri dishes followed by the addition of 20 ml of molten water agar to each plate and mixed well. The agar was allowed to set at room temperature. One set of plates were incubated at 22°C for 72 hours and the other set of plates at 37°C for 24 hours. Appropriate controls for each agar batch are assessed for sterility by pouring an agar plate with no sample for each time and temperature combination used. The colonies on each plate are counted immediately after incubation using an Anderman counter. The results are expressed as the mean number of colony forming units per ml (CFU/ml) of sample computed from the duplicate plates.
In this study we have used the guidelines17 for TVCs recommended for potable (drinking water) quality of 10 CFU/ml at 37°C and 100 CFU/ml at 22°C, although there are no statutory levels for potable water18 we considered these levels a reasonable guide for quality potable water.
Statistical analysis
The microbial count data was entered and analysed in Minitab (version 12). Baseline median counts were compared using a Mann-Whitney test. The log10TVC results from the four outlets investigated were analysed at four time points (Baseline, 1 week, 6 weeks and 13 weeks) using a generalised linear model approach. Follow up comparisons (suitably adjusted for multiple comparisons) were used to identify significant differences between time points.
Results
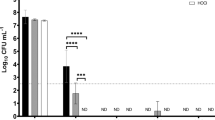
Use of the disinfectant Alpron considerably disrupted the biofilm lining DUWLs as evidenced by the material ejected from dental units after treatment (Fig. 1). The effect of the disinfectant on microbial counts in water from DUWL's are presented in Tables 1, 2, 3, 4. The baseline median TVCs at 22°C from the triple syringe and high speed water lines in the six dental units surveyed were significantly higher than the corresponding counts from tap water supplying the units (P = 0.005).
Using the generalised linear model (Figures 2–5) at time points of baseline, 1 week, 6 weeks and 13 weeks there was a statistically significant drop of 22°C log10TVCs from baseline counts in the water from outlets that had undergone treatment with the Alpron solutions. That is, the triple syringe and high speed water outlets (both P = 0.001), with significant decreases in log10TVC at week 1 and week 6 compared with the baseline for the high speed line and the triple syringe. An analysis of the microbial counts from each of the six units included in the study (Tables 1, 2, 3, 4) demonstrated that most units maintained a pattern of low micro- bial contamination for six weeks. After the six-week period, two units demonstrated TVCs in excess of 100 cfu/ml at weeks 10 and 13. There was no significant change across the untreated control (cup filler) and unit tap water for 22°C TVCs.
Baseline 37°C TVCs from the DUWL outlets revealed a wide range of counts (0-100,000 CFU/ml). There was no significant difference in the 37°C TVCs between the dental unit outlets and the tap water. Following treatment the 37°C TVCs from the majority of the high speed and triple syringe outputs remained at low levels (< 10 CFU/ml) throughout the period of the investigation (Tables 1 and 2). An analysis of the counts from the individual units of the water from the high speed outlets (Tables 1, 2, 3, 4) revealed that at one week post Alpron treatment, two units had 37°C TVCs >10 CFU/ml. By week two all units had 37°C TVCs below 10 CFU/ml. This persisted until 10 weeks post treatment when one unit had 37°C TVCs greater than 10 CFU/ml and at week 13 there were two units that had TVC's >10 CFU/ml. The triple syringe 37°C TVCs fell below 10 CFU/ml after treatment with Alpron solutions and remained below this level for 13 weeks. There was no significant change across the untreated control (cup filler) and unit tap water for 37°C TVCs. No mechanical problems were reported for any of the dental units during the trial period.
Discussion
This study describes the use of a disinfectant solution that appears to disrupt biofilm contaminating dental unit waterlines and maintain low microbial counts over a reasonable time period. The dental units selected for investigation in this study were several years old and had a significant degree of microbial contamination. The units were located in a busy emergency clinic within a dental teaching hospital to closely simulate usage patterns in a general dental practice.
There have been several attempts to reduce the microbial contamination of dental unit water lines including autoclaving of handpieces, handpiece replacement between patients, flushing of the unit prior to use, 'anti-contamination' devices to prevent retrograde aspiration of oral secretions into the water supply line,19 connection to a separate water supply (for example, connection to bottles of distilled water), ultra-violet radiation disinfection and the use of in-line water filters.3,20,21,22 Many workers have suggested treatment with various disinfectant solutions, including hydrogen peroxide,23 chlorhexidine gluconate,24 sodium hypochlorite,25,26 chlorine dioxide,27 povidone–iodine,28 Listerine mouthwash29 and electro-chemically activated water.30 These have been developed and implemented in many dental practices with mixed long-term results. The most commonly used procedure of flushing the handpiece with water prior to use may lower bacterial counts6,15 but high levels of microbial contamination can still persist.6,15,16
The disadvantages of the system described in this report are that it requires a water reservoir for application of the biofilm removal solution. The bottled water system is also necessary for the continual dosing of the water lines to suppress microbial growth and since the water is being used as a coolant, this will result in fine sprays of a weak disinfectant being generated in the dental surgery environment. However the product has achieved the necessary European regulatory clearance for use in this application. Dental units that do not have a water reservoir system would have to have one fitted retrospectively.
The biofilm removal system and disinfectant appears effective in disrupting the biofilm contamination of dental unit waterlines. The results from this study suggest that one course of treatment may be effective for at least 13 weeks under these operating conditions. It may be prudent after 6 weeks to reassess the microbial counts from a treated dental unit in light of the increased counts exhibited by several units at the 10 and 13 week period. In the light of increasing counts the manufacturers recommend a subsequent treatment with the biofilm removal solutions. The manufacturers supply materials for assessing microbial re-growth in dental unit water samples. Whilst the use of Alpron reduced microbial counts in the water supplying the triple syringe and handpieces, we still recommend that sterile water for irrigation should be used for all surgical procedures.
Conclusion
The long-term solution to the control of microbial contamination of DUWLs will depend upon redesigning the water flow through dental units. In the shorter term, disinfectants such as Alpron, may play a role in reducing microbial counts from DUWLs to more acceptable levels.
References
Blake GC . The incidence and control of bacterial infection of dental units and ultrasonic scalers. Br Dent J 1963; 115: 413–416.
Atlas RM, Williams JF, Huntington MK . Legionella contamination of dental unit waters. Appl Environ Microbiol 1995; 61: 1208–1213.
Pankhurst CL, Philpott-Howard JN . The microbiological quality of water in dental chair units. J Hosp Infect 1993; 23: 167–174.
Pankhurst CL, Philpott-Howard JN, Hewitt J, Casewell MW . The efficacy of chlorination and filtration in the control and eradication of Legionella from dental chair water systems. J Hosp Infect 1990; 16: 9–18.
Whitehouse RLS, Peters G, Lizotte J, Lilge C . Influence of biofilms on microbial contamination in dental unit water. J Dent 1991; 19: 290–295.
Williams HN, Kelley J, Folineo D, Williams GC, Hawley CL, Sibiski J . Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. J Am Dent Assoc 1994; 125: 1205–1211.
Williams JF, Johnston AM, Johnson B, Huntington MK, Mackenzie CD . Microbial contamination of dental unit water lines: prevalence, intensity and microbial characteristics. J Am Dent Assoc 1993; 124: 59–65.
Williams HN, Paszko-Kolva C, Shahamat M, Palmer C, Pettis C, Kelley T . Molecular techniques reveal high prevalence of Legionella in dental units. J Am Dent Assoc 1996; 127: 1188–1193.
Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD . Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Micro 2000; 8: 3363–3367.
Reinthaler FF, Mascher F, Stunzer D . Serological examination for antibodies against Legionella species in dental personnel. J Dent Res 1988; 67: 942–943.
Martin MV . The significance of bacterial contamination of dental unit water systems. Br Dent J 1987; 163: 152–154.
Williams JF, Molinari JA, Andrews N . Microbial contamination of dental unit water lines: prevalence, intensity and microbiological characteristics. J Am Dent Assoc 1996; 124: 59–65.
Witt S, Hart P . Cross infection hazards associated with the use of pumice in dental laboratories. J Dent 1990; 18: 281–283.
British Dental Association. Infection Control in Dentistry: Advice Sheet 2000; A12: 7.
Gross A, Devine MJ, Cutright DE . Microbial contamination of dental units and ultrasonic scalers. J Periodontol 1976; 47: 670–673.
Challacombe SJ, Fernandes LL . Detecting Legionella pneumophila in water systems: A comparison of various dental units. J Am Dent Assoc 1995; 126: 603–608.
Standing Committee of Analysts Great Britain Department of Health. The Microbiology of Water 1994. Part 1 - Drinking Water. London: HMSO 1994.
European Union Council directive 98/83/EC on the quality of water intended for human consumption. Off J Euro Comm 1998; L330: 52–54.
Fayle SA, Pollard MA . Decontamination of dental unit water systems: A review of current recommendations. Br Dent J 1996; 181: 369–372.
Scheid RC, Kim CK, Bright JS, Whitely MS, Rosen S . Reduction of microbes in handpieces by flushing before use. J Am Dent Assoc 1982; 105: 658–660.
Scheid RC, Rosen S, Beck FM . Reduction of CFU's in high-speed handpiece water lines over time. Clin Prev Dent 1990; 12: 9–12.
Karpay RI, Puttaiah R, Mills SE, Plamondon TJ, Dove SB, Levine U . Efficacy of flushing dental units for different time periods. J Dent Res 1997; 76 (abstract) 3366.
Kellet M, Holbrook WP . Bacterial contamination of dental handpieces. J Dent 1980; 8: 249–253.
Douglas CWI, Rothwell PS . Evaluation of a dental unit with a built in decontamination system. Quintessence Int 1991; 22: 721–726.
Kim PJ, Cederberg RA, Puttaiah R . A pilot study of two methods for control of dental unit biofilms. Quintessence Int 2000; 31: 41–48.
Abel IC, Miler RL, Micik RE, Ryge G . Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J Dent Res 1971; 50: 1567–1569.
Smith AJ, Bagg J, Hood J . Use of chlorine dioxide to disinfect dental unit water lines. J Hosp Inf 2001; 49: 285–288.
Mills SE, Lauderdale PW, Mayhew RB . Reduction of microbial contamination in dental units with povidone-iodine 10%. J Am Dent Assoc 1986; 113: 280–284.
Meiller T, Baqui A, DePaola L, Overholser CD . Disinfection of dental unit waterlines using Listerine antiseptic. J Dent Res 1995; 74: 153.
Marais JT, Brozel VS . Electro-chemically activated water in dental unit water lines. Br Dent J 1999; 187: 154–158.
Acknowledgements
This study was supported by a grant from Alpron – Quality Water Specialists Ltd
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Smith, A., McHugh, S., Aitken, I. et al. Evaluation of the efficacy of Alpron disinfectant for dental unit water lines. Br Dent J 193, 593–596 (2002). https://doi.org/10.1038/sj.bdj.4801635
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4801635
This article is cited by
-
Contamination of dental unit waterlines: assessment of three continuous water disinfection systems
BDJ Open (2016)
-
Evaluation of microbial contamination and distribution of sulphate-reducing bacteria in dental units
Environmental Monitoring and Assessment (2012)
-
An audit improves the quality of water within the dental unit water lines of general dental practices across the East of England
British Dental Journal (2010)
-
Comparison of the microbial load of incoming and distal outlet waters from dental unit water systems in Istanbul
Environmental Monitoring and Assessment (2009)
-
An audit improves the quality of water within the dental unit water lines of three separate facilities of a United Kingdom NHS Trust
British Dental Journal (2006)